
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
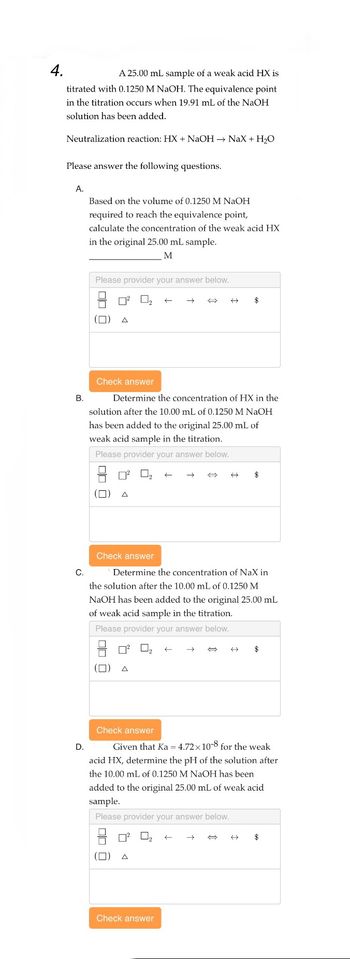
Transcribed Image Text:4.
A 25.00 mL sample of a weak acid HX is
titrated with 0.1250 M NaOH. The equivalence point
in the titration occurs when 19.91 mL of the NaOH
solution has been added.
Neutralization reaction: HX + NaOH → NaX + H₂O
Please answer the following questions.
A.
B.
Based on the volume of 0.1250 M NaOH
required to reach the equivalence point,
calculate the concentration of the weak acid HX
in the original 25.00 mL sample.
M
D.
Please provider your answer below.
0² 0,
(0)
Check answer
Determine the concentration of HX in the
solution after the 10.00 mL of 0.1250 M NaOH
has been added to the original 25.00 mL of
weak acid sample in the titration.
Please provider your answer below.
号
(0)
Check answer
Check answer
C.
Determine the concentration of NaX in
the solution after the 10.00 mL of 0.1250 M
NaOH has been added to the original 25.00 mL
of weak acid sample in the titration.
Please provider your answer below.
0₂
←
A
←
Check answer
$
←
→
← $
Given that Ka = 4.72x10-8 for the weak
acid HX, determine the pH of the solution after
the 10.00 mL of 0.1250 M NaOH has been
added to the original 25.00 mL of weak acid
sample.
Please provider your answer below.
← $
4 $
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- c. What is the concentration of 20.50 mL of NaOH if this was neutralized with 20.0 mL of 0.150 mL HCI in the reaction? NaOH + HCI - NaCI + HOHarrow_forwardConsider the neutralization reaction 2 HNO, (aq) + Ba(OH),(aq) → 2 H,0(1) + Ba(NO,),(aq) A 0.120 L sample of an unknown HNO, solution required 52.3 mL of 0.100 M Ba(OH), for complete neutralization. What is the concentration of the HNO, solution? concentration: Marrow_forwardA 0.124 M NaOH(aq) solution was used to titrate 20.00 mL of an NH3(aq) solution that has an unknown concentration. The equivalence point is reached after adding 22.50 mL of HCl(aq). a. Write out the complete balanced equation for the reaction that occurs in this titration. b. How many moles of HCl were added to the NH3 solution? c. How many moles of NH3 were in the original 20.00 mL solution? d. What was the concentration of NH3 in the original 20.0 mL solution?arrow_forward
- Consider the neutralization reaction 2 HNO,(aq) + Ba(OH),(aq) → 2 H,0(1) + Ba(NO,),(aq) A 0.125 L sample of an unknown HNO, solution required 31.9 mL of 0.200 M Ba(OH), for complete neutralization. What is the concentration of the HNO, solution? concentration: Marrow_forwardWhich of the following precipitates when added to NaOH? a. LiCl b. AgNO3 HBr C. d. Ba(NO3)2arrow_forwardA student performs a titration by combining a 0.890 M hydrobromic acid solution with 65.0 mL of a 0.225 M manganese (III) hydroxide solution. A. Write the complete and balanced equation for the neutralization reaction of hydrobromic acid and manganese (III) hydroxide. DO NOT INCLUDE STATES OF MATTER. B. Calculate the volume of hydrobromic acid, in liters, that was used in this reaction. SHOW ALL WORK FOR FULL CREDIT. T T T F Paragraph 3 (12pt) += - E - T Arial EE T T, @ ABC fx Mashups HTML CSS Path: p Words:0 KAarrow_forward
- How much NaCl should be added to 0.35 L of a 0.15 M solution of AgNO3 so that it reacts completely with the silver to form AgCl(s)? A. 3.07 g OB. 0.0525 g C. 0.89 mg OD. 8.8 garrow_forward20. Consider the complete neutralization reaction of phosphoric acid with calcium hydroxide. How many grams of calcium hydroxide react with 20.2 mL of 0.312 M phosphoric acid.arrow_forwardIt required 20.1 mL of an aqueous HCl solution to react completely with 0.1060 g Na₂CO, (105.99 g/mol). The products are sodium chloride, carbon dioxide, and water. What is the concentration of HCI in the aqueous solution? Select one: O a. 0.0995 M O b. 0.0499 M Oc. 0.0249 M O d. 0.0745 Marrow_forward
- When solutions of Pb2+ and CrO42-are mixed the precipitate PbCrO4 is produced. What volume of 0.1750 M CrO42- removes all lead from 50.00 mL of a 0.3400 M Pb2+ solution? ____ mL of 0.1750 M CrO42- is needed.arrow_forwardB. How many milliliters of 9.95 M hydrochloric acid solution should be used to prepare 4.00 L of 0.300 M HCl? C. Calculate the number of milliters of 0.764 M NaOH required to precipitate as AgOH all of the Ag+ ions in 167 mL of 0.632M AgNO3 solution. D. An aqueous solution of hydrobromic acid is standardized by titration with a 0.169 M solution of barium hydroxide.If 13.0 mL of base are required to neutralize 13.0 mL of the acid, what is the molarity of the hydrobromic acid solution? E. Oxalic acid dihydrate is a solid, diprotic acid that can be used in the laboratory as a primary standard. Its formula is H2C2O4•2H2O.A student dissolves 0.631 grams of H2C2O4•2H2O in water and titrates the resulting solution with a solution of sodium hydroxide of unknown concentration. If 24.1 mL of the sodium hydroxide solution are required to neutralize the acid, what is the molarity of the sodium hydroxide solution ? F. The concentration of Cu+ in a solution is determined by titrating it with a…arrow_forward1. A 12.5 mL volume of 0.500 M H2SO4 neutralizes 50.0 mL of NaOH. A. What is the acid (formula and name)? B. What is the base (formula and name)? C. Write a balanced chemical equation for the reaction. D. Write the complete ionic equation for the reaction. E. Write the net ionic equation for the reaction. F. What is the concentration of the NaOH solution? (This is a titration).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY