
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
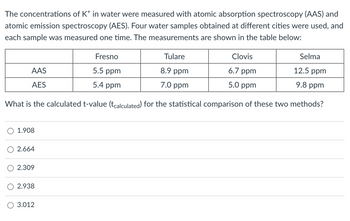
Transcribed Image Text:The concentrations of K* in water were measured with atomic absorption spectroscopy (AAS) and
atomic emission spectroscopy (AES). Four water samples obtained at different cities were used, and
each sample was measured one time. The measurements are shown in the table below:
Tulare
Clovis
8.9 ppm
6.7 ppm
7.0 ppm
5.0 ppm
What is the calculated t-value (tcalculated) for the statistical comparison of these two methods?
AAS
AES
1.908
2.664
2.309
2.938
3.012
Fresno
5.5 ppm
5.4 ppm
Selma
12.5 ppm
9.8 ppm
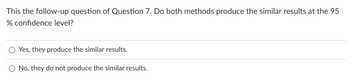
Transcribed Image Text:This the follow-up question of Question 7. Do both methods produce the similar results at the 95
% confidence level?
Yes, they produce the similar results.
No, they do not produce the similar results.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the HQS-Mg fluorescence part of Lab 6, a calibration curve is made of fluorescence intensity vs. MgCl2 concentration (M). The standard solutions for the calibration curve were made by mixing a small amount of 0.01 M MgCl2 with some HQS solution. Calculate the concentration of MgCl2 (M) in each of the following standards. Report your concentrations to 3 sig figs. Fluorescence Volume of 0.01 M Volume of 1 mM Concentration of MgCl2 (M) Intensity MgCl2 (mL) HQS (mL) 0.031250902 0.8 100 0.049490754 0.8 50 0.059803507 2 100 0.085834029 3.6 100 Hint: The total volume of each solution is volume of 0.01 M MgCl2 + volume of 1 mM HQS.arrow_forward10 Find the transition time of 20g naphthalene with the surrounding temperature as 30oC.Let the boiling tube has mass 25 g, diameter 2.5 cm and thickness 0.15cm. How much time does 30g of ice takes to melt at a surrounding temperature of -50C. (We are using a boiling tube having mass= 20g, radius=1.5cm, thickness=0.2cm.) In the above experiment, let the surrounding temperature is changed to 0oC. What will be the change in the transition time for ice? Repeat the experiment for different surrounding temperatures and study the variation of melting point of ice. Calculate the melting point of wax having a mass 30g. Let we are using a boiling tube of mass 25g, having a thickness 0.5 cm and radius 1.5 cm.( Choose surrounding temperature as 40oC)arrow_forwardAtomic absorption spectroscopy was used with the method of standard additions to determine the concentration of cadmium in a sample of an industrial waste stream. For the addition, 10.0 µL of a 1000.0 µg/mL Cd standard was added to 10.0 mL of the unknown solution. The results obtained are shown in the table. Reagent blank Sample Sample plus addition What is the concentration of the cadmium in the waste stream sample? C = Absorbance 0.044 0.300 0.701 C = Later, the analyst learned that the blank was not truly a reagent blank, but water. The absorbance of the actual reagent blank, is 0.10. Calculate the cadmium concentration using the new information for the blank. µg/mL Calculate the percent error caused by using water as the blank instead of the reagent blank. µg/mLarrow_forward
- A 36.0 mg sample of an organic compound (molar mass = 84.0 g/mol) is dissolved in 10.0 mL of water. This aqueous solution is extracted with 5.0 mL of hexane. Separation and analysis of the aqueous phase shows that it now contains 12.0 mg of the organic compound. Calculate the partition coefficient for the compound between hexane and water (Kphexane/water). Your answer should reflect the proper number of significant figures.arrow_forward(5) If 200 g of polymer A, 300 g of polymer B, 500 g polymer C, and 100 g of polymer D are mixed, calculate both M, and Mw of the blend? Polymer A Mn = 45,000; Polymer B M, = 100,000; Mw = 200,000 Polymer C M, = 80,000; Polymer D What are the polydispersity index and the standard deviation of the number distribution of molecular weight of the mixture? Mw = 65,000 Mw = 85,000 Mn = 300,000; Mw = 900,000arrow_forwardUse the data below from the titration of two water samples. Calculate the average ppm for the two trials. Volume of Water sample used for each titration 50.31 Concentration of EDTA used for both trails 0.012 Trial 1 Trial 2 Initial Syringe Volume - 8.98 Initial Syringe Volume - 9.46 Final Syringe Volume - 0.97 Final Syringe Volume - 1.53arrow_forward
- Bradford assay was used to determine bovine serum albumin (BSA) concentrations. The method works by binding Coomassie Brilliant Blue dye to protein standards and unknown, leading to a shift in the absorbance maximum of the dye. A stock solution of BSA is available (10 mg/ml) from which the protein standards were prepared. Bradford reagent, 290 ul, was pipetted into a transparent 96-well microplate. 10 ul of the protein dilution was added followed by mixing in the wells. After 5 min of incubation at room temperature, the plate was read in spectrum mode with the absorbance spectrometer of the microplate reader. The spectrum of the dye not bound to protein was also determined. Create a standard curve or regression equation that related the protein concentration and absorbance. An unknown BSA sample was prepared the same way as the standard and the OD was 0.35. determine the original concentration of the protein solution. The measurements can be seen below. 0.0625 Protein Concentration…arrow_forwardDetermine the mean paracetamol content per tablet (in mg), using the following values: the mean absorbance 0.13, and molar absorption coefficient 9601.9 M−1 cm−1 , where the equivalent half of one tablet mass of powdered paracetamol tablets transferred is 296.4 mg and the mean paracetamol tablet weight is 599.1 mg. Give your answer to TWO decimal places (you do not need to include the units in your answer below).arrow_forwardI have set up my spreadsheet and was able to reproduce the result with a pKa of 4.64. TRUE OR FALSEarrow_forward
- An atomic absorption method for the determination of the amount of iron present in used jet engine oil was found from pooling 30 triplicate analyses to have a standard deviation s = 3.1 µg Fe/mL. If s is a good estimate of o, calculate the 95 and 99% confidence intervals for the result 21.7 µg Fe/mL if it was based on the following criteria. (a) a single analysis 95% confidence interval= 21.7 + 99% confidence interval = 21.7 + (b) the mean of two analyses 95% confidence interval = 21.7 + 99% confidence interval= (c) the mean of six analyses 21.7 ± 95% confidence interval = 21.7 + 99% confidence interval= 21.7 + μg Fe/mL μg Fe/mL μg Fe/mL μg Fe/mL μg Fe/mL μg Fe/mLarrow_forwardTwenty dietary iron tablets with a total mass of 22.131 g were ground and mixed thoroughly. Then 2.998 g of the powder were dissolved in HNO3 and heated to convert all the iron to Fe31. Addition of NH3 precipitated Fe2O3 ? xH2O, which was ignited to give 0.264 g of Fe2O3 (FM 159.69). What is the average mass of FeSO4 ? 7H2O (FM 278.01) in each tablet?arrow_forwardA solution contains 0.10 M Sr(NO3)₂ and 0.20 M Bi(NO₂). When solid Na AsO is added to the solution a precipitate forms. What concentration of AsO ³ maintains maximum separation of Sr²+ and Bi³+? Ksp of Sr₂(AsO₂)₂ = 4.29 x 10-1⁹ and BIASO = 4.43 x 10-1⁰ 4 4/2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY