
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
I need help solving this problem. Greatly appreciate it if you can help me show the step by step process for me to learn

Transcribed Image Text:The calculations outline in the procedure do not account for the warming of the
melted ice to the final temperature. How much energy in joules is required to warm
25.0 grams of water from 0.0'C to 6.2'C? By comparison, melting 25.0 g of ice
requires 8340 J of heat.
Your Answer:
Answer
Expert Solution
arrow_forward
Step 1
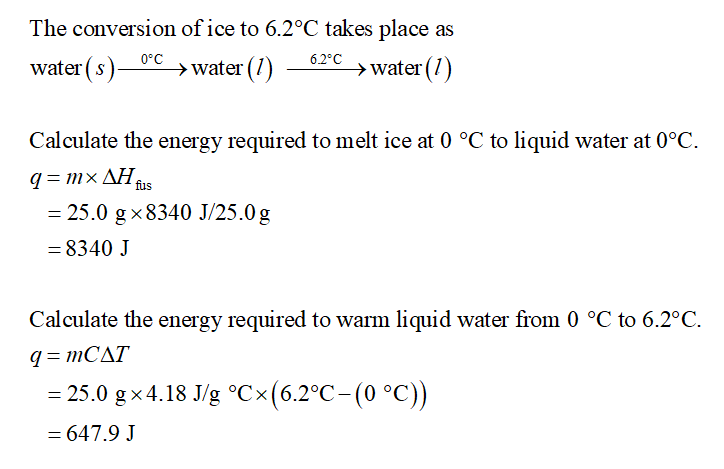
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- To ensure quality solutions, follow these steps: ✔️Submit correct and complete solutions. ✔️Provide step-by-step detailed explanations. ✔️Organize your solution in a clear and structured manner. ✔️Highlight key points and important steps. ✔️Address common points of confusion. By adhering to these guidelines, You can deliver high-quality solutions that are accurate, comprehensive, and easy to understand.arrow_forwardFull Screen O Accessibility A saturated lead(II) chloride solution has a chloride concentration of 3.24 x 10-2 mol/L. What is the Ksp? Put your answer in scientific notation and round to two decimal places. Ksp = Check Answer You can check your answer 4 more times before the question is locked. • Previous MacBook Airarrow_forwardArrange these substance according to how many drops we could add to a penny before the "bubble" popped, FEW DROPS (left-most) to MANY DROPS (right-most). The description labels on the images are small, so use the following legend to identify the materials: A: vegetable oil B: motor oil C: dish soap D: syrup E: honey A CITE EVIDENCE from your Learning Experience and provide REASONING for why you arranged these substances in this order.arrow_forward
- Е. NaCN Br Acetonearrow_forward1a. Determine the volume of 1.000 g of water (Densitywater = 0.9999 g/mL). 1b. Determine the volume of 1.000 g of ice (Density ce = 0.9168 g/cm³). (1 mL = 1 cm³) %3D %3D 1c. From the volumes above, what can we conclude about 1.000 g of water when it freezes?arrow_forwardNewly produced beer must be checked to see if it meets predetermined standards. The percentage of alcohol is determined by distillation. A set of measurements is listed forone batch.Sample Volume of beer (L) Ethanol distilled (mL)1 8.00 3922 10.00 5003 9.00 4594 9.00 444 What is the average percentage by volume of ethanol for this batch?arrow_forward
- 100 B 80 D. 60 40 20 D] 0. 20 30 40 50 60 80 Temperature (C) 50 grams of substance Cis poured into water at 35 degrees.. How many grams won't dissolve? (first determine how many grams will dissolve. then subtract!) 70 10 Solubility (g in 100g water)arrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY