
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
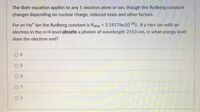
Transcribed Image Text:### The Bohr Equation and Rydberg Constant
The Bohr equation applies to any 1-electron atom or ion, though the Rydberg constant changes depending on nuclear charge, reduced mass, and other factors.
For an He⁺ ion, the Rydberg constant is \( R_{4He} = 2.19176 \times 10^{-18} \, \text{J} \). If a He⁺ ion with an electron in the \( n = 4 \) level **absorbs** a photon of wavelength \( 2153 \, \text{nm} \), in what energy level does the electron end?
**Options:**
- \( \bigcirc \ 6 \)
- \( \bigcirc \ 2 \)
- \( \bigcirc \ 3 \)
- \( \bigcirc \ 7 \)
- \( \bigcirc \ 1 \)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- It is easy to understand how two electrons (one with spin up, one with spin down) can fill the 1s shell for a helium atom. How is it possible that eight more electrons can fit into the 2s2 p level to complete the 1s2s22p6 shell for a neon atom?arrow_forwardQuantum numbers arise naturally from the mathematics used to describe the possible states of an electron in an atom. The four quantum numbers, the principal quantum number (n), the angular momentum quantum number (e), the magnetic quantum number (m,), and the spin quantum number (m,) have strict rules which govern the possible values. Identify all allowable combinations of quantum numbers for an electron. n = 2, l = 1, mę = -1, mş = 1 n = 3, l = 1, mę = 1, mş = - Un = 2, l = 2, mẹ = 0, ms = - n = 5, l = 3, mẹ = -3, ms = - n = 4, l = 2, mẹ = 3, m, = + %3D O n = 3, l = -1, mẹ = 1, m, = -arrow_forwardWhat is the wavelength in nm of a photon emitted from an H atom if an electron transfers from n=6 to n=3 RH = Rydberg constant = 1.097 x 10 -2 nm-1arrow_forward
- what is the wavelength of a line in the calmer series of the hydrogen spectrum, if the transition occurs from ni= 5 to nf = 2 ? remember the rydberg constant is 1.097 x 10^-2 nm^-1arrow_forwardA ground state hydrogen atom absorbs a photon of light having a wavelength of 92.05 nm. What is the final state of the hydrogen atom?arrow_forwardChoose the statement which correctly indicates whether energy is emitted or absorbed and the wavelength of the energy emitted or absorbed (in meters) when the following electronic transition occurs in hydrogen:An electron moves from the n=4 to the n=2 energy level. The value of the Rydberg constant is RH = 1.097 x 107 m-1.arrow_forward
- The energy E of the electron in a hydrogen atom can be calculated from the Bohr formula: E=-Ry/n2 In this equation Ry stands for the Rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron. Calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=5 to an orbital with n=9.arrow_forwardCan you help me answer this question please!arrow_forwardList all possible values of the magnetic quantum number m, for a 4d electron. m₁ = 0 X Sarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY