
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
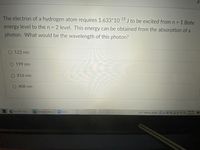
Transcribed Image Text:The electron of a hydrogen atom requires 1.633*10 18 J to be excited from n = 1 Bohr
energy level to the n = 2 level. This energy can be obtained from the absorption of a
photon. What would be the wavelength of this photon?
%3D
O 122 nm
199 nm
O 816 nm
O 408 nm
Quicz HW 3 and 2.
Admissions | Santa.
Zoom
57 F Mostly cloudy
60099
4/18/2022
806 PM
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the energy (in joules) of the line in the spectrum of hydrogen that represents the movement of an electron from Bohr orbit with n = 3 to the orbit with n = 7?arrow_forwardThe energy E of the electron in a hydrogen atom can be calculated from the Bohr formula: R₁ E y 2 n In this equation R, stands for the Rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron. (You can find the value of the Rydberg energy using the Data button on the ALEKS toolbar.) nm Calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transition of the electron from an orbital with n=7 to an orbital with n=2. Round your answer to 3 significant digits. x10 X 3 ? olo 18 Ararrow_forward9. An electron in the hydrogen atom relaxes to the n =2 orbit and 434 nm light is emitted. What was the initial orbit of the electron? A: 5 Me g ou counoearrow_forward
- Is this set of quantum numbers possible? n = 2, l = 1, ml = 0, ms = +1/2arrow_forwardDo number 5arrow_forwardComplete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed. subshell 7p 3d 5s 2s principal quantum number n 1 1 angular momentum quantum number / X Śarrow_forward
- The energy E of the electron in a hydrogen atom can be calculated from the Bohr formula: R n² E In this equation R, stands for the Rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron. (You can find the value of the Rydberg energy using the Data button on the ALEKS toolbar.) Calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transition of the electron from an orbital with n = 7 to an orbital with n=5. Round your answer to 3 significant digits. um Xarrow_forwardThe energy E of the electron in a hydrogen atom can be calculated from the Bohr formula: E=-Ry/n2 In this equation Ry stands for the Rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron. Calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=5 to an orbital with n=9.arrow_forwardWhich principle or rule is violated by the following orbital diagram of an atom in its ground state? 1s 2s 2p For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). 10pt A BIUS Paragraph Arialarrow_forward
- The energy E of the electron in a hydrogen atom can be calculated from the Bohr formula: Ry E 2 n In this equation R, stands for the Rydberg energy, and n Stands for the olo principal quantum number of the orbital that holds the electron. (You can find the value of the Rydberg energy using the Data button on the ALEKS toolbar.) Ar Calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transition of the electron from an orbital with n= 10 to an orbital with n=9. Round your answer to 3 significant digits. O um ?arrow_forward8aarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY