
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
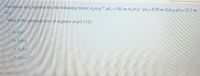
Transcribed Image Text:The amino acid arginine has the following forms: H3Arg* pK, =1.82 H2Arg* pK2=8.99 HArg pK3=12.1 e
Arg
What is the principal form of arginine at pH 11.0?
Oa. Arg
O b. HArg
O c. H2Arg
O d. H3Arg-"
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider a protein in which a negatively charged glutamic acid side chain (pKa=4.2)(pKa=4.2) makes a salt bridge (ion-ion interaction) with a positively charged histidine side chain (pKa=6.5)(pKa=6.5).arrow_forwardWhat are the charges of the following amino acids peptides at ph 14? 1. GLAVV 2. RRKKQarrow_forwardSickle cell anemia is a horrific molecular disease that causes a multitude of issues within the human body, despite the disease’s hemoglobin only slightly varying in structure, as normal hemoglobin’s glutamic acid is instead replaced with valine in the beta-globin amino acid chain. By only changing structurally in this slight amount , sickle cell takes on its unique shape which then causes a multitude of issues within the human body. How does the replacement of glutamic acid with valine end up resulting in this shape, and how would it affect its interaction with the rest of the body?arrow_forward
- Titration of alanine by a strong base, for example NaOH, reveals two pk's. Which is the titration reaction occurring at pK1 (pK1 = %3D 2.34)? O-NH2 + COH →-NH + H2O O-COOH + OH →-CO + H20 -COOH + -NH2 → -COO" + -NH2+ -CoO +-NH2* → -COOH +-NH2 O -NH3* + OH →-NH2 + H2Oarrow_forwarda. How many peptide bonds are there? b. How many amino acid residues are there? c. In exact order, identify the name of the amino acids in the peptide chain. d. Give the full name of this peptide. e. Give the 3-letter abbreviation and 1-letter abbreviation. f. Calculate the pI of this peptide.arrow_forwardThe following data were obtained from partial cleavage and analysis of an octapeptide: Composition: Ala, Gly2, Lys, Met, Ser, Thr, Tyr CNBr: (1) Ala, Gly, Lys, Thr(2) Gly, Met, Ser, Tyr Trypsin: (1) Ala, Gly(2) Gly, Lys, Met, Ser, Thr, Tyr Chymotrypsin: (1) Gly, Tyr(2) Ala, Gly, Lys, Met, Ser, Thr N terminus: Gly C terminus: Gly Determine the sequence of the peptide.arrow_forward
- What is the molecular weight of the Botulinum neurotoxin, a protein that contains 1350 amino acids? Assume an average distribution of amino acidsarrow_forwardWhich of these amino acids is a polar, uncharged amino acid? (Select all that apply, if necessary.) A. B. D. E. CHLO H₂N-CH-C CH₂ CH₂ CH₂ NH C=NH2 NH₂ amino acid A amino acid C amino acid D amino acid B amino acid E H₂N-CH-C CH₂ _i_g C. H₂N-CH-C- CH2₂ I CH₂ =0 NH₂ H₂N-CH- CH₂ CH-CH3 CH3 H₂ND H₂N-CH- | CH₂ Ï OHarrow_forwardDraw the L enantiomer in a Fischer projection for each amino acid; identify the amino acid as neutral, acidic, or basic. Give the three-letter sign [3], and the one-letter symbol [4].arginine a. arginine b. arginine c. a. glutamic acid b. glutamic acid c. glutamic acidtyrosine b. valine c. argininearrow_forward
- Patients suffering from sickle cell anemia have a mutation in the gene that codes for one of the hemoglobin chains, in which a single glutamate is replaced by a valine. Propose an explanation for why this substitution has such a striking effect on protein structure and function with explanation. Suggest two other amino acids that would be less likely to cause such serious impairment of hemoglobin function if each was substituted for glutamate. Briefly explain your answer.arrow_forwardYou are given a protein solution with a concentration of 0.15 mg/ml. Suppose that we want to prepare a solution containing 100 μg of the protein at a concentration of 1 mg/ml. To achieve this, we will first dry down enough protein solution to obtain 100 μg of proteins. How much solution do we need for drying down? How much volume of H2O do we need to add to the dried protein to obtain the desired concentration?arrow_forwardWhich of the following catalyzes reactions that incorporate nitrogen derived from glutamine? a) Glutamine synthase b) Glutamine amidotransferase c) Adenylyltransferase d) Glutamate synthasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON