
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Use E5C.1(a) to Plot the data in excel. Place two data sets on the same graph: one for temp vs. x and one for temp vs y (remember, x and y are the mole fractions and they belong on the x-axis). Don’t forget to include the boiling points of the pure substances – they are data, too.
Add a trendline (polynomial, 3rd order should work) to each of the coexistence lines separately.
Scale the plot appropriately and use the graph to answer the questions.
Then complete E5C.1(b)
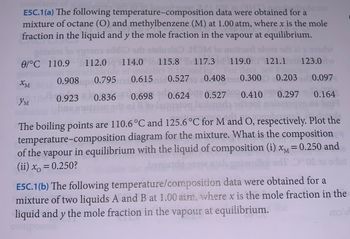
Transcribed Image Text:**E5C.1(a)** The following temperature–composition data were obtained for a mixture of octane (O) and methylbenzene (M) at 1.00 atm, where \( x \) is the mole fraction in the liquid and \( y \) the mole fraction in the vapour at equilibrium.
| θ/°C | 110.9 | 112.0 | 114.0 | 115.8 | 117.3 | 119.0 | 121.1 | 123.0 |
|------|-------|-------|-------|-------|-------|-------|-------|-------|
| \( x_M \) | 0.908 | 0.795 | 0.615 | 0.527 | 0.408 | 0.300 | 0.203 | 0.097 |
| \( y_M \) | 0.923 | 0.836 | 0.698 | 0.624 | 0.527 | 0.410 | 0.297 | 0.164 |
The boiling points are 110.6°C and 125.6°C for M and O, respectively. Plot the temperature–composition diagram for the mixture. What is the composition of the vapour in equilibrium with the liquid of composition (i) \( x_M = 0.250 \) and (ii) \( x_O = 0.250 \)?
**E5C.1(b)** The following temperature/composition data were obtained for a mixture of two liquids A and B at 1.00 atm, where \( x \) is the mole fraction in the liquid and \( y \) the mole fraction in the vapour at equilibrium.
(Note: To fully interpret this data on an educational website, students should be guided on how to plot the temperature-composition diagram and interpret the equilibrium states of the mixtures based on the provided mole fractions and temperatures.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please don't provide handwritten solution ....arrow_forwardi need help with this questionarrow_forwardThe student then determined ΔH neutralization for the reaction of sodium hydroxide and acetic acid, using the procedure described in this module. The student added 100.0 mL of 0.8500M NaOH to 100.0 mL of 0.8404M acetic acid. Prior to and following the mixing of the acid and base solutions, the following temperature-time data were collected. g) Identify the limiting reagent and briefly explain why it is limiting. h) Find ΔH neutralization for the reaction.arrow_forward
- I cannot understand why H2 is being a limiting reactant in this case.arrow_forwardMail- Thomps 3 3 https://app.101edu.co 80 P Parchment Reg F3 $ 4 Write the balanced NET ionic equation for the reaction when AlCl3 and NaOH are mixed in aqueous solution. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction. 1 2 + 04- F4 Na set TW6D2PHI.pdf 2 % ( ) 5 3 4 03 □ F5 Unofficial Transcri 4 Question 15 of 40 ↑ NR O 6 5 05 0+ F6 6 UAFS Registra Graduation Applica MacBook Air & 7 2 2+ (s) (1) CI Al 7 8 9 0 09 口。 3+ 7 04+ F7 8 (g) (aq) • x H₂O 8 DII F8 H My UAFS-St Delete DD F9 M 0arrow_forwardKsp= 3.00 x 10^-17arrow_forward
- Enthalpy of the Neutralization DeltaHn in kj/mol Average deltaHn in kj/mol arrow_forwardCalculate the solubility at 25 °C of BaCrO4 in pure water and in a 0.0170M BaCl₂ solution. You'll find data in the ALEKS Data tab. sp Round both of your answers to 2 significant digits. solubility in pure water: 0² 09 solubility in 0.0170 M BaCl₂ solution:: 0.2 X OxO 00 Śarrow_forwardCalculate the solubility at 25 °C of CuBr in pure water and in a 0.0140 M CoBr, solution. You'll find Ksp data in the ALEKS Data tab. Round both of your answers to 2 significant digits. solubility in pure water: solubility in 0.0140 M CoBry solution: 品 品arrow_forward
- Please need help with this first question , not sure of the change in temperaturearrow_forwardFor the following reactions, predict whether the mole fraction of the reactants or products increases or remains the same when the volume of the reaction vessel is increased. SO2 (9) + 1/2 0, (9) = SO3 (g) а. mole fraction of reactants increases mole fraction of products increases mole fraction remains the same 2 CH4 (g) = C2H2 (g) + 3 H2 (g) b. mole fraction of reactants increases mole fraction of products increases Omole fraction remains the same N2 (9) + 2 O2 (9)=2 NO2 (g) с. mole fraction of reactants increases mole fraction of products increases Omole fraction remains the samearrow_forward6. Two units of non-atomic traffic travel from start to end in the selfish routing network shown below. There are three paths. (a) Find the equilibrium flow and cost. (b) Find the optimal flow and cost and use this and your answer in part (a) to calculate the POA.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY