
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
Knowing the mass of sodium bicarbonate used, one can calculate the moles
of sodium bicarbonate and the moles of HCl. With the volume readings from the burette and the moles of HCl
calculated, the molarity of the HCl solution can be found.
Na2CO3 is mixed with 25 mL of water in the titrated flask. final reading - Initial of HCl in burette is amount used. Show work
A.) determine the concentration of the HCl solution from the data for the standardization of the HCl with the Na2CO3.
B.)calculate the moles and mass of acetylsalicylic acid in each tablet (molar mass = 180.16 g/mol).
C.)calculate the % by mass of aspirin in the tablet.
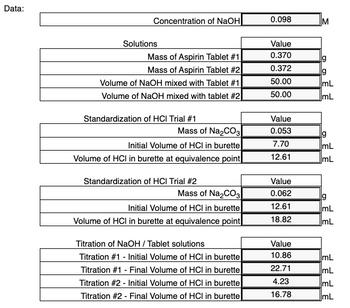
Transcribed Image Text:Data:
Concentration of NaOH
Solutions
Mass of Aspirin Tablet #1
Mass of Aspirin Tablet #2
Volume of NaOH mixed with Tablet #1
Volume of NaOH mixed with tablet #2
Standardization of HCI Trial #1
Mass of Na₂CO3
Initial Volume of HCI in burette
Volume of HCI in burette at equivalence point
Standardization of HCI Trial #2
Mass of Na₂CO3
Initial Volume of HCI in burette
Volume of HCI in burette at equivalence point
Titration of NaOH / Tablet solutions
Titration #1 - Initial Volume of HCI in burette
Titration #1 - Final Volume of HCI in burette
Titration #2 - Initial Volume of HCI in burette
Titration #2 - Final Volume of HCI in burette
0.098
Value
0.370
0.372
50.00
50.00
Value
0.053
7.70
12.61
Value
0.062
12.61
18.82
Value
10.86
22.71
4.23
16.78
g
mL
ImL
g
mL
ImL
g
mL
mL
mL
mL
mL
mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4arrow_forwardAcid-Base titration: Standardization of 0.1 M NAOH Trial 1 Mass KHP used, g 0.297 g Final reading Volume NaOH, mL 15.59 mL Initial reading Volume NaOH, mL 0.23 mL Volume NaOH consumed, mL 15.36 mL Moles KHP used Moles NaOH used Molarity of NaOH solutionarrow_forwardMolarity of the standard solution NaOH is 0.0994Marrow_forward
- Ans. 17.15 mg. 54) A 1.000-g sample of a mixture which contains only NaCl and KCl gare a precipitate of AgCl which weighed 2.000 g. What are the percentages of Na and K in the mixture? ins 10 g Ans. 44.77% K; 5.75% Na. 1:J 55. I- can be sernarated from othorarrow_forwardThe following graphs represent the behavior of BaCO3 underdifferent circumstances. In each case, the vertical axisindicates the solubility of the BaCO3 and the horizontalaxis represents the concentration of some other reagent.(a) Which graph represents what happens to the solubilityof BaCO3 as HNO3 is added? (b) Which graph representswhat happens to the BaCO3 solubility as Na2CO3 is added?(c) Which represents what happens to the BaCO3 solubilityas NaNO3 is added? [arrow_forwardQ2. At 25°C, water dissolves 0.8108g of PbCl2 per liter, calculate the Ksp of PbCl2 at 25°C. -12 ilitarrow_forward
- o/index.html?deploymentld%=55750828934189288909969212&eiSBN=9781305657571&snapshotld%32199898&id%3... ☆ IDTAP Q Search this cou Use the References to access important values if needed for this question. The concentration of H3ASO3 in a solution is determined by titration with a 0.1945 M Ce+ solution. The balanced net ionic equation for the reaction is: 2Ce*(aq) + H3ASO3(aq) + 5H2O(1) 2Ce*(aq) + H3ASO4(aq) + 2H3O*(aq) (a) If 19.83 mL of the 0.1945 M Ce** solution are needed to react completely with 30.00 mL of the H3ASO3 solution, what is the concentration of the H3ASO3 solution? M (b) Which of the two solutions was in the buret during the titration? (c) Suppose at the end of the titration, the solution containing the Ce³* ion is transferred to a volumetric flask and diluted to 300. mL. What is the concentration of Ce3+In the diluted solution? Submit Answer 5 question attempts remainingarrow_forward30mL 0.9% v/v green food coloring. Transfer 2mL of of food coloring to flask, QS the flask to 100mL with DI water. What is the concentration of the standard?arrow_forwardWhat is the easiest process to get to the result?arrow_forward
- 10. A 0.514 gram sample of NazCO, (106.0 g/mol) was dissolved in distilled water in a 100.0 mi volumetric flask. The molar concentration of Na,CO, in solution is: 0.0485 M 0.0370 M b. 0.4849 M 0.0340 M 0.0330 M d. е.arrow_forward3.A sample is known to contain NaOH, Na2CO3 NaHCO3 or compatible mixture of these together with inert matter. With methyl orange, a 1.100 g sample requires 31.40 mL of HCl ( 1.00 mL is equivalent to 0.0140g CaO). With phenolphthalein indicator, the same weight of sample requires 13.30mL of the acid. What is the percent composition of the sample? ww wwarrow_forwardSolid cobalt (II) acetate is slowly added to 125 mL of 0.0945 M ammonium chromate solution. What is the concentration of cobalt required to just initiate precipitation? The Ksp of CoCrO4 is 7.1 * 10 -4. Report answer in scientific notation to two sig figs.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
