
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:Chrome
M Gmail
esc
File Edit
G Google
=
X
YouTube Maps
View History
0
Galeks login- X
www-awu.aleks.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-lvTqeviKFP6W0cqJcWJdIACROQwyw24GW
C Rank the ele X
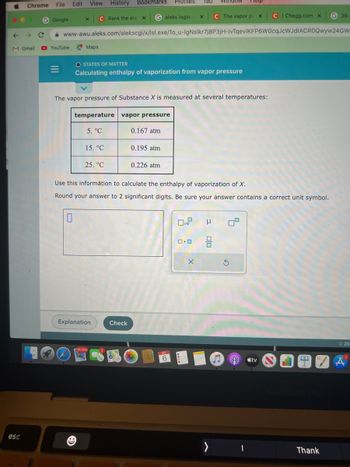
temperature
5. °C
O STATES OF MATTER
Calculating enthalpy of vaporization from vapor pressure
15. °C
The vapor pressure of Substance X is measured at several temperatures:
25. °C
Profiles
Bookmarks
Explanation
16,500
vapor pressure
0.167 atm
Check
0.195 atm
0.226 atm
Use this information to calculate the enthalpy of vaporization of X.
Round your answer to 2 significant digits. Be sure your answer contains a correct unit symbol.
X
Tab window
SEP
6
x10
X
C The vapor pr. X
μ
0.0 2
>
Heip
8.
5
C | Chegg.com x
e tv
I
C
G 39
Thank
©20
A
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 3.7 atm. pressure (atm) 0- 8 solid liquid gas 100 temperature (K) 200 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. ☐ °C Garrow_forwardLL The vapor pressure of Substance X is measured at several temperatures: temperature vapor pressure -27. °C 0.0449 atm -16. °C 0.112 atm -5.°C 0.257 atm Use this information to calculate the enthalpy of vaporization of X. Round your answer to 2 significant digits. Be sure your answer contains a correct unit symbol. Continue O 2022 McGraw Hill LLC. All Rights Re EPIC MacBook Pro ロ号 F3 F4 F5 F2 24 4. 23 7. 6. 3. 5. A G D. B. N 8arrow_forwardConsider a liquid with a vapor pressure of 200. torr at 20.0 °C and a vapor pressure of 400. torr at 50.0 °C. What is the closest value to the normal boiling point of this liquid? 83.8 °C O 357 °C O 295 °C 18.2 °C 22.0 °Carrow_forward
- How to calculate the boiling point of methylene chloride at 670mm Hg? (need some hint)arrow_forwardkJ The enthalpy of vaporization of Substance X is 10.0 and its normal boiling point is 110. °C. Calculate the vapor pressure of X at 33. °C. mol Round your answer to 2 significant digits. atm х10arrow_forwardA pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results: temperature (°C) 160. 140. 120. 100. 80. 60. 0. 10. What is the boiling point of X ? Use this graph to answer the following questions: heat added (kJ/mol) 20. What phase (physical state) of X would you expect to find in the flask after 12 kJ/mol of heat has been added? 30. Пос (check all that apply) solid O liquid gas 40.arrow_forward
- Substance X is known to exist at 1atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other properties of X have been determined (picture). You may also assume X behaves as an ideal gas in the vapor phase. Suppose a small sample of X at -20°C is put into an evacuated flask and heated at a constant rate until 10 kJ/mol of heat has been added to the sample. Graph the temperature of the sample that would be observed during this experiment.arrow_forwardThe vapor pressure of Substance X is measured at several temperatures: temperature vapor pressure -98. °C 0.0231 atm -85. °C 0.0964 atm -72. °C 0.334atm Use this information to calculate the enthalpy of vaporization of X. Round your answer to 2 significant digits. Be sure your answer contains a correct unit symbol. x10arrow_forwardQ1.)Using the data provided in Table 3 in the handout (also provided below), calculate the vapor pressure of water at 21.0 °C.?? Table 3: Vapor pressure of water at various temperatures T (˚C) P (mmHg) T (˚C) P (mmHg) T (˚C) P (mmHg) 0 4.58 16 13.63 26 25.21 5 6.54 18 15.48 28 28.35 10 9.21 20 17.54 30 31.82 12 10.52 22 19.83 40 55.3 14 11.99 24 22.38 50 92.5 PART B) In the experiment, you wrap a piece of copper wire around your magnesium strip to suspend the magnesium inside the eudiometer. Why is copper wire a good choice for this task? MULTIPLE CHOICE : A.It conducts electricity very well B. It reacts strongly with the acid C. It does not react with the acid D. It is much heavier than Mgarrow_forward
- Try Again Your answer is incorrect. The enthalpy of vaporization of Substance X is 23.0 Round your answer to 2 significant digits. 0.32 atm x10 X kJ and its normal boiling point is 10. °C. Calculate the vapor pressure of Xat - 18. °C. mol Sarrow_forwardA sample of C3H4Br2 has a normal boiling temperature of 131.5 °C. The enthalpy of vaporization for this compound is 35.4 kJ/mol. What would be the boiling temperature in °C of C3H4Br2 at a pressure of 540.0 mm Hg? Report your answer using three significant figures.arrow_forwardUse the observation in the first column to answer the question in the second column. observation The enthalpy of vaporization of Substance E is bigger than that of Substance F. At 36 °C, Substance C has a vapor pressure of 111. torr and Substance D has a vapor pressure of 91. torr. At 1 atm pressure, Substance A boils at 36. °C and Substance B boils at 52. °C. question Which has the higher boiling point? Substance E Substance F Neither, E and F have the same boiling point. It's impossible to know without more information. Which has a higher boiling point? Substance C Substance D Neither, C and D have the same boiling point. It's impossible to know without more information. Which has a higher enthalpy of vaporization? Substance A Substance B Neither, A and B have the same enthalpy of vaporization. It's impossible to know without more information.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY