
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
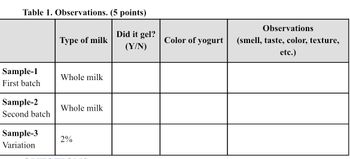
Transcribed Image Text:Table 1. Observations. (5 points)
Observations
Type of milk
Did it gel?
(Y/N)
Color of yogurt
(smell, taste, color, texture,
etc.)
Sample-1
Whole milk
First batch
Sample-2
Whole milk
Second batch
Sample-3
Variation
2%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Density of solution:Trial 1: 1.2 g/mLTrial 2: 1.2 g/mLTrial 3: 1.2 g/mL Average density = 1.2 g/mL What is the relative average deviaion, %?arrow_forward7) You have a prescription for 150g of 2% psoriasis w/w cream but only 8% cream in stock. How much of this more concentrated cream is required to make this prescription?Units- garrow_forwardBoth A &B pleasearrow_forward
- SELECT correct responses only 1) Volume percent (v/v%) is temperature-independent. 2) There are three major approaches to expressing solution concentration in terms of percent concentration: weight percent, volume percent, and weight/volume percent. 3) Weight/volume percent (w/v%) is a unit-less quantity. 4) The term 'percent' refers to 'parts per thousand'. 5)A standard solution is a reagent of known concentration that is used to carry out a volumetric titration. 6) The endpoint of a titration is the theoretical point reached when the amount of added titrant is chemically equivalent to the amount of analyte in a sample. 7) A substance that changes color in response to chemical equivalence is known as an indicator. 8) Given a sufficiently diligent and experienced experimenter, it is sometimes possible to reach a titration volume error value of 0.arrow_forwardment Score: n 5 of 26 0% How many grams of dextrose are needed to make 745 mL of a 16.0% (w/v) dextrose solution? Note that mass is not technically the same thing as weight, but the abbreviation % (m/v) is often used interchangeably with % (w/v). mass:arrow_forwardAnswer questions 1 and 2 according to the data given. Dater Weight of cream i: 326 kỳ. Table shows some data obtained directly from Lactascan instrument. Fat % SNF % Lacto- density 27.32 30.23 Raw milk 4.15 7.97 Skim milk 0.08 8.26 Q1. Calculate fat percent of creamn A) 46 B) 28 (C) 3D D) 0.046 آدرما 396 100 x Q2. Calculate efficiency of the cream separator. A) 100% B98% C) 97% D) 96% E) 87.2% amount 2500 L E) 0.28arrow_forward
- i need help calculating slope and inverse slope pleasarrow_forwardA dilute aqueous solution containing 1 ppm of solute has a density of 1.00 g/ mL. Express the concentration of solute in g/L, g/mL, and mg/ L. (Show work/calculations)arrow_forwardA sample of rainwater was collected from four separate locations across theMetroplex. Each sample was measured once by two separate methods for the presence ofsulfate. Determine whether the two methods are equivalent with respect to giving thesame answer for [SO42-].arrow_forward
- Need help finding the concentrations for each solutionarrow_forwardratio stuength (vv)? 2. f a pharmacist added 12 g of azelaic acid to 50 g of an ointment containing 15% azelaic acid, what would be the final con- centration of azelaic acid in the ointment? -ki solution werearrow_forward12:27 2) milliliters 13.10 TEMU B. Convert 1.90 gallons to each of the following. Show your set ups. elszovee Report answers in decimal notations with the proper number of significant figure. 1) liters 3) quarts NJD Send a Chat ||| O P 44% اللهarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning



Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
