
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
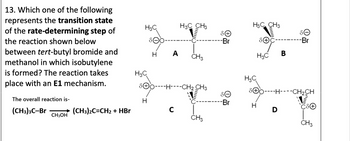
Transcribed Image Text:13. Which one of the following
represents the transition state
of the rate-determining step of
the reaction shown below
between tert-butyl bromide and
methanol in which isobutylene
is formed? The reaction takes
place with an E1 mechanism.
The overall reaction is-
H3C
H3C CH3
H3C CH3
Omm
Θό
-Br
Ꮎ
-Br
H A
CH3
H3C
B
H3C
H3C
SOOH-CH2 CH3
SOHCH2CH
H
-Br
H
(CH3)3C-Br
(CH3)2C=CH2 + HBr
с
D
CH3OH
CH3
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- How many transition states and intermediates would the reaction profile have for the reaction shown below?arrow_forward4. Outline a detailed mechanism for the reaction below. Identify the abbreviation for the reaction mechanism. НО H3PO4arrow_forwardChloromethane reacts with dilute sodium cyanide (Na+ -C‚N) chloromethane cyanide acetonitrile chloride. When the concentration of chloromethane is doubled, the rate is observed to double. When the concentration of cyanide ion is tripled, the rate is observed to triple. Write the rate equation for this reaction.arrow_forward
- Consider the following reaction mechanism. Step 1: 2X + Y- W + 2 Z fast Step 2: Z- U+ V slow Step 3: V+Z-2 W + Y fast The rate-limiting step is Step because. 1; the first step must occur before any others can occur O 2; it uses a product of the first step O 3; the last step is always the rate-limiting step O 2; it is the slowest steparrow_forwardIdentify the transition state for the reaction between tert-butyl chloride and sodium hydroxide. H H-c-H 8- 8- HO---- C- CI H3C CH3 H 8- Но- -H----c-H 8- HạC-C- ČH3 -CI H-c-H 8+ 8+ HO---- CI H3C CH3 H. &+ HO-----H----C 8+ H3C-C----CI ČH3 %3arrow_forward3. The following three SNAR transformations follow different mechanisms. Draw an intermediate or transition state for each reaction that relates the correct mechanism. Write a short sentence as to why that is the correct intermediate for the reaction. Reaction 1: Reaction 2: NO2 NO2 NO₂ + NaOMe NO2 + NaOH2 1. aq NaHCO3. 100 C 2. H₂O+ OMe NO2 NO₂ OH ŇO₂ NO2 Reaction 3: CFa Br CF3 8--2 + NaNH2 NH3 NH₂ Intermediate/transition state Intermediate/transition state Intermediate/transition statearrow_forward
- Below is the equation for a nucleophilic substitution reaction and some experimental data. CH3CH2Br + CH3COO- ⇌ CH3CH2CO2CH3 + Br- ΔH=-75 kJ/mol Rate = k [CH3CH2Br][CH3COO-] Which reaction energy profile would be the best representative of the data provided?arrow_forwardWhich of the species below best depicts the likely transition state for the reaction between methyl iodide, CH3I and sodium methylthiolate, NaSCH3?arrow_forwardPlease draw the detailed mechanism of this below reaction scheme in 2 steps. Please draw it carefully, I don't understand, for example, why in the first step, the C = O is replaced by C = N and in the second step, the 2 double bonds can react with each other.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning