
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Suppose the formation of nitrosyl chloride proceeds by the following mechanism:
step elementary reaction rate constant
(g) (g) (g)
(g) (g) (g)
Suppose also ≫. That is, the first step is much faster than the second.
Write the balanced chemical equation for the overall chemical reaction :
Write the experimentally-observable rate law for the overall chemical reaction.
Note: your answer should not contain the concentrations of any intermediates.
Express the rate constant k for the overall chemical reaction in terms of k1, k2, and (if necessary) the rate constants k-1 and k-2 for the reverse of the two elementary reactions in the mechanism.
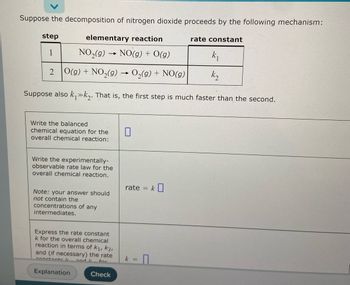
Transcribed Image Text:Suppose the decomposition of nitrogen dioxide proceeds by the following mechanism:
step
elementary reaction
rate constant
1
NO₂(g)
→
NO(g) + O(g)
k₁
2 O(g) + NO₂(g) → O₂(g) + NO(g)
k₂
Suppose also k₁»k₂. That is, the first step is much faster than the second.
Write the balanced
0
chemical equation for the
overall chemical reaction:
Write the experimentally-
observable rate law for the
overall chemical reaction.
Note: your answer should
not contain the
concentrations of any
intermediates.
Express the rate constant
k for the overall chemical
reaction in terms of K₁, K2,
and (if necessary) the rate
and I
for
Check
constants l
Explanation
rate = k
k =
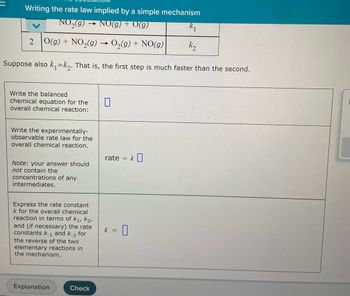
Transcribed Image Text:Writing the rate law implied by a simple mechanism
NO₂(g) → NO(g) + V(g)
k₁
2 O(g) + NO₂(g) → O₂(g) + NO(g)
k₂
Suppose also k₁k₂. That is, the first step is much faster than the second.
Write the balanced
chemical equation for the
overall chemical reaction:
0
Write the experimentally-
observable rate law for the
overall chemical reaction.
Note: your answer should
not contain the
concentrations of any
intermediates.
Express the rate constant
k for the overall chemical
reaction in terms of K₁, K2,
and (if necessary) the rate
constants k-1 and k-2 for
the reverse of the two
elementary reactions in
the mechanism.
Explanation
Check
rate =
k =
0
k0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the gas phase decomposition of t-butyl chloride, (CH3)3CC (CH3)2C=CH2 + HCI the rate constant in s has been determined at several temperatures. When In k is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.08×104 K and a y-intercept of 28.6. The value of the rate constant for the gas phase decomposition of t-butyl chloride at 582 K is (Enter your answer to one significant figure.)arrow_forwardA study of the rate of dimerization of CH, gave the data shown in the table. 2 C4H6 → C3H12 Time (s) 1800. 3600. 5400. 7200. [CH,] (M) 9.98x10-3 4.74x10-3 3.11x10-3 2.32x10-3 1.84x10-3 (a) Determine the average rate of dimerization (in M•s) between 0 s and 1800. s. 4.0 M.s-1arrow_forwardQuestion 17 of 19 Submit What is the rate law for the proposed mechanism? A) rate = k[H2][NO] H2(g) + 2NO(g) → N20(g) + H2O(g) B) rate = k[Hzl°[NO] Step 1 (slow) C) rate = k[H2][NO]? N20(g) + H2(g) N2(g) + H2O(g) Step 2 (fast) D) rate = k[H2l°[NO]? E) rate = k[H2][N2O]arrow_forward
- 2) Use the data below to determine the rate law and the value of k for the following reaction H2O2 (aq) + 3 I (aq) + 2H* (aq) →13° (aq) + H20 (1) Experiment [H20z] (M) [F] (M) [H*] (M) Initial Rate 0.0390 0.0470 0.0309 5.149 x 10-6 0.0779 0.0470 0.0309 1.030 x 10-5 2.059 x 10-5 5.149 x 10-6 3 0.0390 0.0939 0.0309 4 0.0390 0.0470 0.0620arrow_forwardSolve all three parts otherwise I will downvote...arrow_forwardDetermine the rate law for the formation of oxygen predicted by this mechanism. (Assume the steady-state approximation is valid for any reaction intermediates.) k₁ CIO + CIO + M (CIO)₂ → CIOO + Cl (CIO)2 + sunlight k3 C100 + M k4 →→ Cl + O₂ 2x (C1 + 03 net: 2 033 0₂ → CIO + O₂) (this reaction occurs twice in the mechanism)arrow_forward
- (a) In the reaction mechanisms given, there are some chemical species which will get produced in one step and get consumed in the other step and that are not in reactants nor in products of the total reaction. Such species are known as intermediates of a reaction as the name suggests. Therefore, in mechanism 1, the intermediates are (CH3)2CHOH2+, Cl-, (CH3)2CH+. and in mechanism 2: (CH3)2CHOH2+, Cl-. These ions are neither reactants nor products of the given total equation.arrow_forwardd) The integrated rate law for a reaction, describes how the reaction rate depends on the concentration of reactants. True Falsearrow_forwardSuppose the decomposition of ozone proceeds by the following mechanism: step elementary reaction rate constant (g) (g) (g) (g) (g) (g) Suppose also ≫. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentally-observable rate law for the overall chemical reaction. Note: your answer should not contain the concentrations of any intermediates. Express the rate constant k for the overall chemical reaction in terms of k1, k2, and (if necessary) the rate constants k-1 and k-2 for the reverse of the two elementary reactions in the mechanism.arrow_forward
- Consider this reaction: 2H₂PO4 (aq) → P₂O5 (aq) + 3H₂O (aq) At a certain temperature it obeys this rate law. rate = (0.069 M¹.¹) [H₂PO4] Suppose a vessel contains H₂PO4 at a concentration of 0.380 M. Calculate how long it takes for the concentration of H3PO4 to decrease to 5.0% of its initial value. You may assume no other reaction is important. Round your answer to 2 significant digits. ? Submit A Continue 2 Xarrow_forwardDetermine the rate law for the reaction, 2IC1 + H2 = I, + 2HCi, from the following initial rate data: Initial Rate(Ms') 2.04x102 4.08x102 2.55x103 5.09x103 [H,], [ICI], 0.250 0.500 0.500 0.500 0.125 0.125 0.125 0.250 A) R = k[IC1]? В) R = k[H2]? C) R = k[ICI][H_]? D) R = k[ICl][H,] E) R = k[ICI]°[H¿] 000arrow_forwardFor the gas phase decomposition of ethyl acetate, CH;COOC,H5–CH;COOH + C,H4 the rate constant in s1 has been determined at several temperatures. When In k is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.41×10“ K and a y-intercept of 28.8. The value of the rate constant for the gas phase decomposition of ethyl s1. acetate at 675 K is (Enter your answer to one significant figure.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY