
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
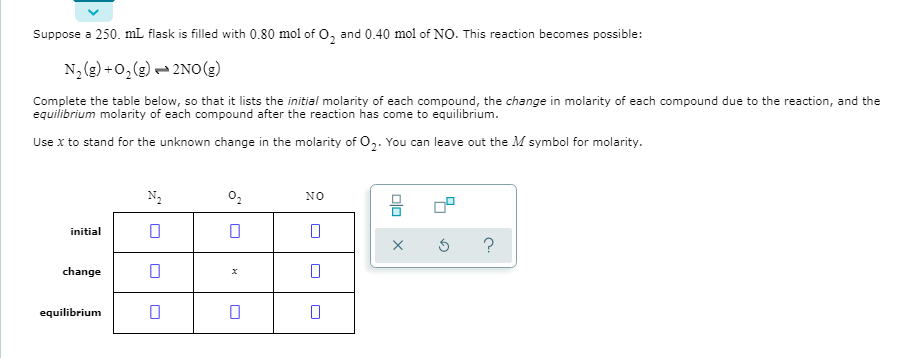
Transcribed Image Text:Suppose a 250. mL flask is filled with 0.80 mol of O, and 0.40 mol of NO. This reaction becomes possible:
N, (g) +0, (g) - 2NO(g)
Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the
equilibrium molarity of each compound after the reaction has come to equilibrium.
Use x to stand for the unknown change in the molarity of 0,. You can leave out the M symbol for molarity.
N2
02
NO
initial
change
equilibrium
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Suppose a 250. mL flask is filled with 1.5 mol of CO, 0.50 mol of H₂O and 1.2 mol of CO₂. The following reaction becomes possible: CO(g) +H₂O(g) CO₂(g) +H₂(g) The equilibrium constant K for this reaction is 0.503 at the temperature of the flask. Calculate the equilibrium molarity of CO. Round your answer to two decimal places. M ܢܢ X E 00 Ararrow_forwardSuppose a 250. mL flask is filled with 0.10 mol of N, and 1.8 mol of NO. The following reaction becomes possible: N2(g) + 0,(g) == 2NO (g) The equilibrium constant K for this reaction is 0.422 at the temperature of the flask. Calculate the equilibrium molarity of O2. Round your answer to two decimal places. IM ?arrow_forwardSuppose a 250. mL flask is filled with 1.1 mol of CO, 0.80 mol of H,O and 0.60 mol of CO,. The following reaction becomes possible: CO(g) +H,0(g) - CO,(g)+H2(g) The equilibrium constant K for this reaction is 5.72 at the temperature of the flask. Calculate the equilibrium molarity of CO. Round your answer to two decimal places. | Marrow_forward
- Suppose a 500. mL flask is filled with 1.6 mol of N₂ and 0.40 mol of NO. The following reaction becomes possible: N₂(g) + O₂(g) 2NO(g) The equilibrium constant K for this reaction is 6.10 at the temperature of the flask. Calculate the equilibrium molarity of N₂. Round your answer to two decimal places. M X Śarrow_forwardConsider the following reaction: 2NH3 (9) N2(g) + 3H₂ (9) = If 0.00148 moles of NH3(g), 0.268 moles of N₂, and 0.643 moles of H₂ are at equilibrium in a 11.7 L container at 932 K, the value of the equilibrium constant, Ke, isarrow_forwardSuppose a 250. mL flask is filled with 1.5 mol of CO, 0.80 mol of NO and 0.70 mol of CO,. The following reaction becomes possible: 2' NO, (g) +CO(g) - NO(g)+CO,(g) The equilibrium constant K for this reaction is 6.92 at the temperature of the flask. Calculate the equilibrium molarity of NO. Round your answer to two decimal places. olo Ar OM ?arrow_forward
- Suppose a 500. mL flask is filled with 0.30 mol of SO, and 1.2 mol of SO3. This reaction becomes possible: 2SO, (g) +O,(g) 2SO3 Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of O,. You can leave out the M symbol for molarity. so, SO2 O2 initial change equilibriumarrow_forwardWhile ethanol (CH,CH,OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH,CH,) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 100 L tank with 45. mol of ethylene gas and 35. mol of water vapor. When the mixture has come to equilibrium he determines that it contains 34. mol of ethylene gas and 24. mol of water vapor. The engineer then adds another 18. mol of water, and allows the mixture to come to equilibrium again. Calculate the moles of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. | molarrow_forwardWhile ethanol (CH,CH,OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH,CH,) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 50 L tank with 15. mol of ethylene gas and 22. mol of water vapor. When the mixture has come to equilibrium he determines that it contains 7.8 mol of ethylene gas and 14.8 mol of water vapor. The engineer then adds another 5.5 mol of water, and allows the mixture to come to equilibrium again. Calculate the moles of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. |molarrow_forward
- Suppose a 500. mL flask is filled with 1.4 mol of N, and 1.3 mol of NO. This reaction becomes possible: N,(2) +0,(2)- -2NO(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of N,. You can leave out the M symbol for molarity. N2 O2 NO initial change equilibrium oloarrow_forwardAfter equilibrium is reached in the reaction of 6.30 g H2 with 150. g I2, analysis shows that the 1.00 L flask contains 64.0 g of HI. How many moles of H2, I2, and HI are present in this equilibrium mixture? What is the Keq for this reaction?arrow_forwardSuppose a 250. mL flask is filled with 1.4 mol of CO, 1.9 mol of H₂O and 1.0 mol of CO₂. The following reaction becomes possible: CO(g) + H₂O(g) + CO₂(g) + H₂(g) 2 The equilibrium constant K for this reaction is 9.28 at the temperature of the flask. Calculate the equilibrium molarity of CO. Round your answer to two decimal places. M X Ś ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY