
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Please do not round off intermediate calculations. Thank you.
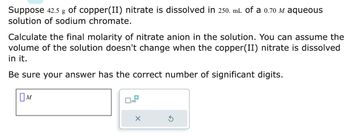
Transcribed Image Text:Suppose 42.5 g of copper(II) nitrate is dissolved in 250. mL of a 0.70 M aqueous
solution of sodium chromate.
Calculate the final molarity of nitrate anion in the solution. You can assume the
volume of the solution doesn't change when the copper(II) nitrate is dissolved
in it.
Be sure your answer has the correct number of significant digits.
M
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Content Google Do google slid X Inbox (4,5 ✓ Certificate × > Course E X Cengage L xb Will a prec ✓ OWLv2|0 × ChatGPT ✓ + C 口 New Chrome available : prod03-cnow-owl.cengagenow.com/ilrn/takeAssignment/takeCovale Press to exit full screen take (fn) F to exit full s This question has multiple parts. Work all the parts to get the most points. [References] E4 CH 16 17 and 18 Question 1 1 pt Question 2 2 pts Question 3 1 pt Question 4 2 pts Question 5 1 pt a Consider the reaction of NO and Cl₂ to produce NOCI. 2 NO(g) + Cl2(g) → 2 NOCl(g) What is AS (system) for this reaction? Question 6 × 2 pts Compound S° (J/mol K) . NO(g) 210.76 Question 7 1 pt Question 8 2 pts Cl2(g) NOCI(g) 223.08 261.8 Question 9 1 pt AS (system) = . J/K • mol-rxn Question 10 2 pts Question 11 1 pt Submit Question 12 1 pt Question 13 1 pt Submit Answer Try Another Version 2 item attempts remaining Question 14 1 pt Question 15 1 pt Progress: 9/15 items Due Apr 14 at 05:00 PM Finish Assignment Cengage Learning | Cengage…arrow_forwardMission 2: Experiment- Tools and Techniques in Analytical Measurements Datasheet for 37 A-1: Using the Analytical Balance Measurement (s) Weight of Penny (g) [by Method 1] Weight of Penny (g) [by Method 2] Penny 1 2.48 2.51 Penny 2 2.49 2.50 Penny 3 2.50 2.52 2.49 2.48 Penny 4 Penny 5 2.51 2.47 From the above data, you will determine the mean and median values, the standard deviation, and the relative standard deviation of the masses of the pennies.arrow_forwardNWP Assessment Player UI Appli X WP -> A https://education.wiley.com/was/ui/v2/assessment-player/index.html?launchld=eb66b5b0-1212-4354-98f4-985dc387b4b3... В NEW E Apps P Pearson Sign In Sign in to your acco... E Set 04 - SP22 Question 11 of 23 - / 1 Draw an organobromide that will react with lithium diphenylcuprate to give the following compound: OCH3 Draw Your Solution 10:48 PM O Type here to search 30°F a ») ENG 1/29/2022arrow_forward
- Is the precision of your two trials within 5 %?. Support your answer with calculationsarrow_forwardThank you! One of this was incorrect according to the platform I was uploading my homework inarrow_forwardMacmillan Learm [S] (mM) 1.5 Product formed (umol min) 0.21 2.0 0.24 3.0 0.28 4.0 0.33 8.0 0.40 16.0 0.45 Calculate the standard error of regression (SER) for the Lineweaver-Burk slope and the Eadie-Hofstee slope to compare the precision of each. 0.14 Lineweaver-Burk SER: Incorrect 0.017 Eadie-Hofstee SER: Incorrect umol min μmol minarrow_forward
- Chrome File Edit View History Bookmarks Profiles Tab Window Help 96% WPAL 101_ 23 Purple/Black I x O Gloss Purple G $550 mustang X O S550 Mustanc X X dt8827@unc Dementia Frie x ALEKS - Davi A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZI6tTytly4Fcfu6zOtOf80MM9sefhQp9QAYI8idN-02kvSt27lmROEJa_z.. D Pa O CHEMICAL REACTIONS Solving for a reactant using a chemical equation - 0/5 A major component of gasoline is octane (C3H18). When octane is burned in air, it chemically reacts with oxygen gas (O2) to produce carbon dioxide (CO2) and water (H,). What mass of oxygen gas is consumed by the reaction of 2.26 g of octane? Be sure your answer has the correct number of significant digits. ?arrow_forwardbard 1 ! New folder M Reading Mode: 1.5... M Gmail ض A- ش X Course Home Z~ is 2 https://openvellum.ecollege.com/course.html?courseld=17485264&OpenVellumHMAC-453c4e9377366ab5782f501c5246fbe0#10001 ▾ 2 Part A alt W= VA 3 Part B Complete previous part(s) S- = Part C Complete previous part(s) Part D Complete previous part(s) X a 2,4-floro, 5-methyl he XI Determine if each of the following cycloalkanes and alkenes can exist as cis-trans stereoisomers. Drag the appropriate items to their respective bins. Cis-trans isomers are possible. Submit Request Answer XO, F3 # 3 E- [» "! D[ LS ra. YouTube 4 C { $ R 24 F1 H₂C. Maps FS ▬▬▬ ▬▬ % 2,4-fluoro,5-methyl h X CH₂ 5 V} TY CH₂ ف GY Br. CH₂ 6 Ą Cis-frans isomers are not possible. XXX YI Copyright © 2022 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy Permissions | Contact Us | BY Y 5-Methyl-2,4-hexane X m.__ CH₂CH₂CH-CHF & 7 Hi FB U E W J NI Pearson ی 8 F9 DELL Reset Help Q trimethyl - prt sc 1 ÷ D MA ( 9 к. 30 F10 ن O…arrow_forwardDATA/CALCULATION SHEET NAME 1. crucible, cover, BaCl2 2H2O \$,2061 crucible and cover 14.0188g BaCl2 2H2O 4.1873 8 first second third weighing weighing weighing crucible, cover, anhydrous BaCl2 17,5815 H2O lost (use your last weighing) 17.58158 SHOW CALCULATION % water, experimental (See prelab) % water, theoretical SHOW CALCULATION % errorarrow_forward
- & Spring 2023 CHEM 1411 14 General Chemistry I LAB Experiment 2 Postlab 57 minutes remaining Postlab Content Page 3 of 6 Question 2 An object with a mass of 47.2 kilograms and a volume of 47.6 liters. Find the density with units of g/cm³. Report your answer with three significant figures. Add your answer Questions Filter (5) 5 OF 5 QUESTIONS REMAINING K First Last > Continue Details & In PER Assessm 2/23/23 X ✓ • You after 76°F Time li 60 min Attem 2 atte Grading Saarrow_forwardThis question was rejected, but this question is part of a practice set and as you can see it is out of zero points. Other practice questions from this set were answered.arrow_forwardme x C Thermo X Cosmet x C Laptops x M Inbox (7 X C Buy TCL X learn.canterbury.ac.uk/ultra/courses/_19016_1/outline/edit/document/_3537106_1?courseld=_19016_1&view=content T K Saturat X ZA a Amazon x C Show Ye X G what's a X Email- 1 X ☆ b The dia x h EX E ct the rato at which + 4. Consider the interconversion of A and B. Suppose in the absence of an enzyme, the forward rate constant KF is 104 s¹ and the reverse rate constant KR is 106 S¹. Calculate the equilibrium constant K. How would the presence of an enzyme affect the value of K? Karrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY