
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I need help on this?
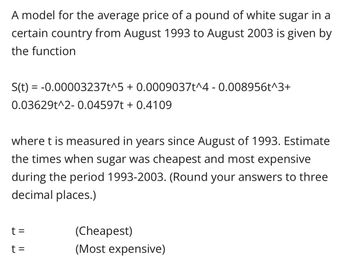
Transcribed Image Text:A model for the average price of a pound of white sugar in a
certain country from August 1993 to August 2003 is given by
the function
S(t) = -0.00003237t^5 + 0.0009037t^4 -0.008956t^3+
0.03629t^2- 0.04597t+ 0.4109
where t is measured in years since August of 1993. Estimate
the times when sugar was cheapest and most expensive
during the period 1993-2003. (Round your answers to three
decimal places.)
t =
t =
(Cheapest)
(Most expensive)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Two liquids, A and B are immicible. Liquid A has a density of 0.89 g/mL. Liquid B has a density of 1.00 g/mL. What would you expect to see if you added equal amounts of both liquids to a test tube, shook, and let settle? a. one clear layer b. one cloudy layer c. two layers with A on top d. two layers with B on toparrow_forwardThe active ingredient of Benadryl© Chesty Forte Cough Liquid is Guaiphenesin anexpectorant drug used to assist the expectoration ('bringing up') of phlegm from theairways in acute respiratory tract infections. A 200.0 mL bottle of Benadryl© ChestyForte Cough contains 4.00 g of Guaiphenesin (C10H14O4). i. If the recommended dose for a child is 150 mg of Guaiphenesin. What volume of thecough liquid should be administered?arrow_forwardKw=1.0x10-¹4 Report all pH values to two places past the decimal. 1. Write the following acid/base reactions. Circle the bases and underline the acids. a. hypochlorous acid (HCIO) reacts with water b. the base ammonia (NH3) reacts with waterarrow_forward
- A good sample of benzoic acid melts at 121-122˚C. However, a student had a sample that melted over a range of 114 to 118 ˚C. What did the student conclude about this sample?arrow_forwardI don't know this question?arrow_forwardA 1185 mL sample of drinking water was found to contain 20.5 mg of lead. Calculate the concentration of lead in milligrams per liter. concentration: mg/L MacBook Air トト F11 F12 888 FB F7 F3 F4 24 & * 4 5 6 7 8 Y Karrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY