
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
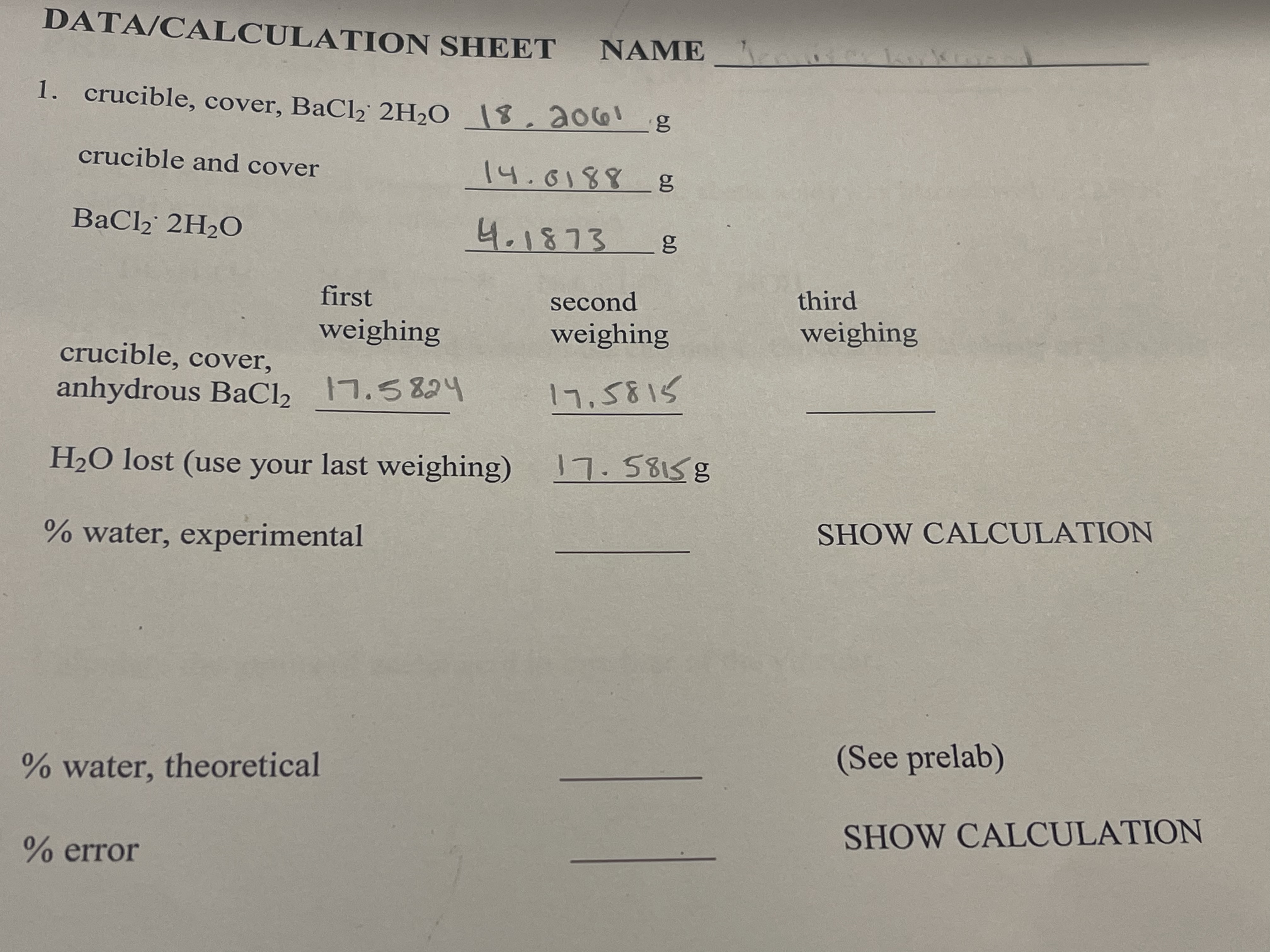
Transcribed Image Text:DATA/CALCULATION SHEET NAME
1. crucible, cover, BaCl2 2H2O \$,2061
crucible and cover
14.0188g
BaCl2 2H2O
4.1873 8
first
second
third
weighing
weighing
weighing
crucible, cover,
anhydrous BaCl2
17,5815
H2O lost (use your last weighing)
17.58158
SHOW CALCULATION
% water, experimental
(See prelab)
% water, theoretical
SHOW CALCULATION
% error
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- /1wdGaF3fc41TY-3NU4JhvIQJLK2kFekLX/edit minutes ago IUA 三 ==,E 三E|X 20 2 | E 3 LI 4 IT3 E OI I 7 + EXPERIMENT Percent vield Due after completing the lab. Answer in the space provided. 1. A student reacted 0.500 g of Pb(NO.)2 with 0.750 g of KI according to the reaction, Pb(NO.b (aq) + 2 KI (aq)- → Pbb (s) +2 KNO: (aq) How many moles of Pb(NO.)2 were used? mol of Pb(NO) b) How many moles of KI were used? mol of KI c) How many moles of PbL would form, based on mol of Pbb the moles of Pb(N0) used? d) How many moles of Pbl would form, based on mol of Pbl the moles of KI used? e) Which is the limiting reactant? What is the theoretical yiekd of PbL in grams? 主 g of PbL g) If the student obtained 0.583 grams of PbL product after collecting it by fihration and dry ing it, what was the percent yield of Pbl obtained? 2. Diborane, B H, can be produced by the following reaction: NaBH (aq) H 50 (a) = H (g) = Na SO (aql= BH(B) What is the maximum quantity, in grams, of B H that can be prepared…arrow_forwardHAPTER 3 - CHEMICAL REACTIONS Study Page 1 of 3 Next O References Use the References to access important values if needed for this question. hen the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: sulfur (s) + carbon monoxide (g) sulfur dioxide (g) + carbon (s) sp.cengage.info/als-asp/take a 80°F O Type here to search Caarrow_forwardmistry app.101edu.co YouTube Maps NG AMOUNT * @ 7 2 X W S F2 X Jill # Welcome to MyTCC Welcome to MyTCC 3 F3 E D C 20: $ 4 R F 30 % 35.6 V Aluminium has a density of 2.70 g/cm² How many moles of aluminum are in a 13.2 cm block of the metal substance? 4 ADD FACTOR X x() F5 5 molecules Al T Content G 1.32 F6 A 6 B 2.70 0.489 g Al/mol F7 Y & H Question 1 of 50 0.100 g/cm² W 7 26.98 N ASUS X F8 U J g Al Lo b Home | bartleby ANSWER 8 962 13.2 F9 | M mol Al 1 1 K F10 9 < I RESET 5 6.022 x 10 O cm 1 F11 O L X + 10 P : F12 - I Prt Sc 1 ? + [ Q 12 ☆ = 12 Insert 1 Delete Backspace J Submit Home Enter PgUp Shift Pearrow_forward
- Imole 1,5g Cu 504,3 X Imole ('u 5 0415H20 = Moles of anhydride used. (Show calculations) = Cu Soy • 5H20(s) A ('u 504 (5) + 5H20 (9) Hydrate » anhydride Moles of H₂O of hydration (Show calculations)= Ratio of moles of water to moles of anhydride (Show calculations.) = Molarmass = (to correct sig. figs. - do not round yet) Based on your data, what is the empirical formula of your hydrate (round your ratio to the closest whole number)? Use the Argumentation Framework (see inside front cover) (Claim- Evidence- Justification) to justify your answer. 147 S 112 52arrow_forwardVolume (mL) Trial 1 Trial 2 Trial 3 Final volume (mL) 24.35 47.60 20.60 Initial volume (mL) 1.25 25.15 42.85 Volume Added 23.10 22.45 22.35 Average Volume Added 22.63 mL Concentration of NaOH 0.9971 M % Acetic Acid in Vinegar Assuming all the acid to be acetic, calculate the number of grams of acid per 100 mL of vinegar solution. Assuming that the density of vinegar is 1.000, what is the percentage of acetic acid by weight in vinegar? Average your results in the usual manner. % of Acetic Acid = (mL of NaOH added)(concentration of NaOh)/mL of vinegar samplearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY