
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
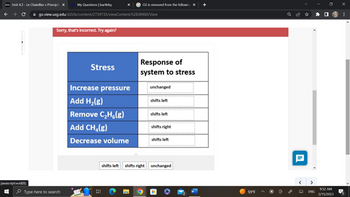
The following system is at equilibrium in a closed vessel:
2 CH4(g) ⇆ C2H6(g) + H2(g)
Various stresses are applied to the system as illustrated in the chart. Drag the appropriate label from the list below that indicates how the system responds to each stress. Each label can be used more than once.
Sorry, that's incorrect. Try again?
Transcribed Image Text:Unit 42-Le Chateliers Principle X
My Questions | bartleby
go.view.usg.edu/d21/le/content/2739735/viewContent/52838900/View
javascript:void(0
Sorry, that's incorrect. Try again?
Type here to search
Stress
Increase pressure
Add H₂(g)
Remove C₂H₂(g)
Add CH₂(g)
Decrease volume
02 is removed from the followin
Response of
system to stress
unchanged
shifts left
shifts left
shifts right
shifts left
shifts left shifts right unchanged
59°F
I
ENG
952 AM
2/15/2023
Expert Solution
arrow_forward
Step 1

Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- "Synthesis gas" is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas will form carbon dioxide and hydrogen, and in fact this reaction is one of the ways hydrogen is made industrially. A chemical engineer studying this reaction fills a 5.0 L flask with 1.0 atm of carbon monoxide gas and 2.5 atm of water vapor. When the mixture has come to equilibrium she determines that it contains 0.69 atm of carbon monoxide gas, 2.19 atm of water vapor and 0.31 atm of hydrogen gas. The engineer then adds another 0.25 atm of carbon monoxide, and allows the mixture to come to equilibrium again. Calculate the pressure of carbon dioxide after equilibrium is reached the second time. Round your answer to 2 significant digits. atmarrow_forwardNitrogen dioxide is one of the many oxides of nitrogen (often collectively called "NOx") that are of interest to atmospheric chemistry. It can react with itself to form another form of NOx, dinitrogen tetroxide. A chemical engineer studying this reaction fills a 2.0 L flask with 1.6 atm of nitrogen dioxide gas. When the mixture has come to equilibrium she determines that it contains 1.1 atm of nitrogen dioxide gas. The engineer then adds another 0.80 atm of nitrogen dioxide, and allows the mixture to come to equilibrium again. Calculate the pressure of dinitrogen tetroxide after equilibrium is reached the second time. Round your answer to 2 significant digits.arrow_forward"Synthesis gas" is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas will form carbon dioxide and hydrogen, and in fact this reaction is one of the ways hydrogen is made industrially. A chemical engineer studying this reaction fills a 2.0 L flask with 3.1 atm of carbon monoxide gas and 3.6 atm of water vapor. When the mixture has come to equilibrium she determines that it contains 1.0 atm of carbon monoxide gas, 1.5 atm of water vapor and 2.1 atm of carbon dioxide. The engineer then adds another 0.90 atm of water, and allows the mixture to come to equilibrium again. Calculate the pressure of hydrogen after equilibrium is reached the second time. Round your answer to 2 significant digits. 0 atm x10 C [arrow_forward
- While ethanol (CH3CH,OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH,CH,) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 500. mL flask with 0.77 atm of ethylene gas and 0.98 atm of water vapor. When the mixture has come to equilibrium he determines that it contains 0.34 atm of ethylene gas and 0.55 atm of water vapor. The engineer then adds another 0.19 atm of ethylene, and allows the mixture to come to equilibrium again. Calculate the pressure of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. atmarrow_forwardAt a certain temperature, the reaction: PCl5(g) ⇆ PCl3(g) + Cl2(g) has an equilibrium constant Kc = 6.7E-6. Calculate the equilibrium concentration of PCl3 if only PCl5 is present initially at a concentration of 0.30 M. Your answer should have two significant figures.arrow_forwardAt a certain temperature, the reaction: PCl5(g) ⇆ PCl3(g) + Cl2(g) has an equilibrium constant Kc = 6.7E-6. Calculate the equilibrium concentration of PCl3 if only PCl5 is present initially at a concentration of 0.30 M. Your answer should have two significant figures.arrow_forward
- A closed 1.00 L system initially containing 0.00200 mol H₂ and 0.00500 mol 1₂ at a given temperature is allowed to reach equilibrium Analysis of the equilibrium mixture shows that the amount of HI present at equilibrium is 0.00371 mol. Calculate the Kc at this temperature for the reaction. State whether the equilibrium is product-favored or reactant- favored at this temperature. H₂(g) +1₂(g) 2H1(g) Kc 31: The equilibrium is product-favored. Kc 31: The equilibrium is reactant-favored. Kc 0.032; The equilibrium is reactant-favored. Kc - 0.032; The equilibrium is product-favored.arrow_forwardPhosphorous pentachloride decomposes according to the reaction PCl5(g)↽−−⇀PCl3(g)+Cl2(g)PCl5(g)↽−−⇀PCl3(g)+Cl2(g) A 13.1 gsample of PCl5 is added to a sealed 1.25 L flask and the reaction is allowed to come to equilibrium at a constant temperature. At equilibrium, 48.4% of the PCl5 remains. What is the equilibrium constant, Kc , for the reaction?arrow_forwardMethane and water react to form carbon monoxide and hydrogen, like this: CH2(g)+H₂O(g) CO(g)+3H2(g) Suppose a mixture of CH 4, H2O, CO and H2 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation change in composition shift in equilibrium to the right The pressure of CH4 will ? to the left Some CO is added. The pressure of H2O will ? (none) to the right The pressure of H2O will ? Some CH4 is removed. to the left The pressure of CO will ? (none)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY