
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Give detailed Solution with explanation needed..give correct answer
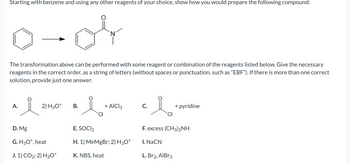
Transcribed Image Text:Starting with benzene and using any other reagents of your choice, show how you would prepare the following compound:
0-04
The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary
reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct
solution, provide just one answer.
A.
i
2) H3O+
D. Mg
G. H3O+, heat
J. 1) CO2; 2) H3O+
B.
N
i
+ AICI 3
E. SOCI₂
H. 1) MeMgBr; 2) H3O+
K. NBS, heat
C.
CI
+ pyridine
F. excess (CH3)2NH
I. NaCN
L. Br2, AlBr3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the pH of a 0.553 M weak base solution? The Kb of the weak base is 0.000013. Your Answer: Answerarrow_forwardWhat is the pH of a 0.049 M weak base solution? The Kb of the weak base is 0.000018. Your Answer: Answerarrow_forwardPlease circle answer and workout on paper I need it for studyingarrow_forward
- What is the pH of a 0.391 M weak base solution? The K, of the weak base is 0.000077. Your Answer: Answerarrow_forwardExample Problem: Consider The Titration of 100.0 mL of 0.200 M CH3NH2 by 0.100 M HCl. Calculate The pH After 65.0 mL of HCl Added. (Kb for CH3NH2 = 4.4 x 10-4): a. 10.96 b. 9.75 c. 7.00 d. 3.68 e. 2.54arrow_forwardPlease correct answer and don't use hend raitingarrow_forward
- What is the pH of 500ml solution conating 0.125 M fornic acid and 0.140 M sodium formate? Include chemical equation What would the pH be if 1mL of 0.5 HBr was added to solution? pka=3.744arrow_forwardIs caffeine an acidic or basic compound? Answer using a balanced equilibrium equation for caffeine in water that includes the correct structure for caffeine.arrow_forwardcan search the web by typing the chemical name followed by SDS) b) what is the best practice to follow if a small amount of sulfuric acid (1M) comes in contact with your bare hand? 2. Write a one or two sentence statement of what the purpose of this lab is. 3. To prepare for laboratory: For each of the eight unknowns solutions that you will identify in lab (see above or table below), identify the chemical color and the pH category (acidic, neutral, or basic) that you expect to find. Prepare a table to summarize your initial expectations for the color and pH tests. A. Introduction- A Fictitious Scenario ne Chem-Police, a division of the Office of Homeland Security created in re-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY