
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
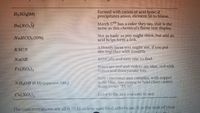
Transcribed Image Text:HSO,(IM)
Formed with cation of acid fame; if
precipitates anion, element 56 to blame.
Ba(NO,
March 17 has a color they say, that is the
same as this chemical's flame test display
NaHCO, (10%)
Not as basic as you might think, but add an
acid helps foTm a link.
A bloody mess you might see, if you put
this together with iron(III).
KSCN
NaOH
BASICally and easy one to find.
Fe(NO,),
Roses are red and violets are blue, red with
itmus and thtocyanate to0.
Very emotional and compleX, with copper
quite blue, toO Strong to hold close causes
many to say "PLW"
NILOH (6 M) (aqueous NH).
Cu(NO,),
Coloris the key and casy to see.
The concentrations are all 0.25 M unless specified otherwise. It. s the task of your

Transcribed Image Text:can search the web by typing the chemical name followed by SDS) b) what is
the best practice to follow if a small amount of sulfuric acid (1M) comes in
contact with your bare hand?
2. Write a one or two sentence statement of what the purpose of this lab is.
3. To prepare for laboratory: For each of the eight unknowns solutions that you
will identify in lab (see above or table below), identify the chemical color and
the pH category (acidic, neutral, or basic) that you expect to find. Prepare a
table to summarize your initial expectations for the color and pH tests.
A. Introduction- A Fictitious Scenario
ne Chem-Police, a division of the Office of Homeland Security created in re-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardFor each of the pH values listed in the first column, calculate and record the corresponding hydronium and hydroxide concentrations.arrow_forwardWhat is the [H+], in moles per liter, in a solution with a pH of 2.500? Enter your answer to three significant figures. Do not include unitsarrow_forward
- A solution contains 0.133 M NH4Cl and 0.199 M ammonia. The pH of this solution is Submit Answer Retry Entire Group 2 more group attempts remainingarrow_forwardPart A Reset Help significant When determining the pH of a polyprotic acid solution or mixture of acids, only the [OH | acid needs to be considered. Any another other ionizations are by comparison, and ion content. the overall negligible must be considered in does not contribute to strongest weakest [H;O]+arrow_forwardIf the pH of a solution is 3.3, then the pOH is ( Select ] and the solution is [ Select ]arrow_forward
- 8) Hydrofluoric acid Use Ka and Kb values from the equation sheet provided CHEM_III_Eqn_Sheet How many grams of HF must be dissolved in water to create 604 mL of a solution with a pH of 2.47? Mass of HF 4.094e-2 Evaluate Change Problem Values Incorrect. Hint: find the concentration of the hydrofluoric acid in solution, given the pH. Solving some of the previous problearrow_forward[References] Use the References to access important values if needed for this question. An aqueous solution contains 0.444 M hypochlorous acid. Calculate the pH of the solution after the addition of 3.15x10-2 moles of potassium hydroxide to 225 mL of this solution. (Assume that the volume does not change upon adding potassium hydroxide). pH = Submit Answer Retry Entire Group 6 more group attempts remaining Previous Email Instructor Next Savearrow_forwardUse the References to acc An aqueous solution has a hydrogen ion concentration of 1.0 × 10-10 M. What is the hydroxide ion concentration in this solution? Concentration = | M Is this solution acidic, basic or neutral? Submit Answer Retry Entire Group 3 more group attempts remainingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY