
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Give the reaction mechanism for the image below
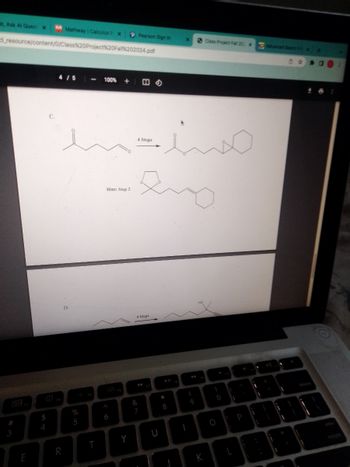
Transcribed Image Text:st, Ask Al Quest X
Mathway | Calculus Px
Pearson Sign In
X
Class Project Fall 202 x Advanced Search x
d_resource/content/0/Class%20Project%20Fall%202024.pdf
C.
4/5
100%
+
20
Hint: Step 2
4 Steps
inso
-end
D.
4 Steps
%
85
64
#3
E
R
+11
&
6
7
8
T
Y
U
4
61
1
K
O
481
delete
L
enter
return
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- O Rich * Ceng x вЫ Еxcel Bb What X O covI x A Daily x O Amon x e Chat x Conte x A ALEK X A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsIkr7j8P3jH-liJOkWvnm4w-aQ-rw-zRhgRnayfmbBs65spEMBxW_NZIUBEIMJCK O Mail - Ava Schied.. O UAConnect e Biology Syllabus Labflow - Courses S Explore - HogSync e Packback Apps B Blackboard Question 2 Objective Knowledge Check Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: some ionic compounds cation anion empirical formula name of compound Co 3+ so, Co2+ 10, Zn2+ Bro Ba2+ PO I Don't Know Submit O 2020 McGraw-Hi. MacBook Proarrow_forwardHing.com Get?Fileld-hNDLmdusbEmplc8ue%2laZn4otcFEREdCkEOBEgn%2bHNOn13v5HvafUMjlu77SxrKT vity - Saved to itslearning P Search Layout References Review View Help O Editing v w Ro... v 12 A^ U ev Av A =<而< 向 川< 3Ba+ N2 → BazN2 7. Which element is your limiting reactant Barium or Nitride? (Hint: which produced less product) Pb(NO3)2 + 2KI → PbI, + 2KNO3 8. How many moles are in 16.4g of Lead (II) nitrate? Pb(NO3)2 + 2KI → PbI2 + 2KNO3 9. How many moles of lead (II) iodide are formed from your moles of lead (II) nitrate in question 8? Pb(NO3)2 + 2KI → PbI, + 2KNO3 10. How many grams are there in your moles of lead (II) iodide from question 9? Pb(NO3)2 + 2KI → PbI2 + 2KNO3 11. How many moles are in 28.5g of potassium iodide? 909 U.S.) Text Predictions: On ASUS f10 (12 f5 f6 f8 f9 & 6 7 8 因arrow_forwardS Physical Science: Q2: 2021-22 (L X S Abigail Spencer | Physical Scienc x MAlg. 1-ldentifying Linear Functior x A alleneast schoolsplp.com/enrollments/172020834/items/7ab47cea-18be-4119-95c8-3ab6b5397059/work Abigail Spencer Next Activity > Physical Science: Q2: 2021-22 (LL): Mastery Assess It 4 All changes saved A 17. You leave a half-full water bottle in the car in the heat of summer. The next time you get in the car, you notice condensation inside the water bottle. This is an example of O synthesis O chemical equilibrium O physical equilibrium an open system > N PREVIOUS 17 of 25 NEXT REVIEW SAVE & EXIT DELL %24 & 林 Warrow_forward
- K Haylee Scott - cp Zoom meeting lin com/web/viewer.html?state%=D%7B'ids %3A%5B"1zOflgo1GoQFrdviJ6PWx8L3h2j4uav5n"%5D%2C"action"%3A'open C Clever | Portal A Classroom https://sso.theleam. M Oro Grande Elemen. W Yearbook Aven ue Period 5 Scien. Haylee Scott - cprr061.pdf Building Vocabulary From the list below, choose the term that best completes each sentence. matter physical change endothermic reaction chemical change chemistry precipitate exothermic reaction 7. Any change that alters a substance without changing it into another substance is a(n) 8. is anything that has mass and takes up space. 9. A reaction that releases energy in the form of heat is called a(n) 10. A(n) absorbed. 11. A chemical reaction is also referred to as a(n) is a reaction in which energy is 12. A(n) a chemical reaction. is a solid formed from a solution during 13. is the study of the properties of matter and how matter changes. OPearson Education, Inc., publishing as Pearson Prentice Hall. All rights reserved…arrow_forwardTab Minbox (1,600)-fantilOudeledu X Mail- Francesca A Tantillo-Out xEXP #12: Geometry-CHM150-2x Aktiv Chemistry ← → C < Esc app.101edu.co Mostly sunny O ! D 7 A Silane, SiH., is a colorless, pyrophoric, toxic gas that is used to deposit elemental silicon for semiconductor and photovoltaic applications. Given the electron configuration of Si is [Ne]3s 3p2, how many valence electrons does Si have? Z 2 W S X # 3 E D $ 4 C OL R F % 5 FS T Question 18.b of 23 V G ^ 6 Y B & 7 H PrtScn U N 8 J Home ( 9 M K X End O 1 4 7 +/- PgUp 0 2 5 8 1. P 3 - 6 9 0 Pgon $12 0 APD + Update Submit C x 100 5:21 PM 7/6/2022 X 10 Del Backspacearrow_forwardChrome File Edit View History Bookmarks People Tab Window Help HOc Dashbc X E Buy Es: X G find so X © Periodi x A ALEKS X M Mathwa X Hcc Chapte x H New m x ト→ C A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQİHQRDYV_6Ux63SypJXz0Coxvwqgg4JkWI7Cge0xmXkyh... O GASES Using Charles's Law Jacqueline An arctic weather balloon is filled with 48.4 L of helium gas inside a prep shed. The temperature inside the shed is 9. °C. The balloon is then taken outside, where the temperature is -42. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Round your answer to 3 significant digits.arrow_forward
- M Inbox (321) - jjuhasz@ X in Course: NTR-3230-130 X My Courses Course Home Syllabus Scores Pearson eText Study Area Document Sharing User Settings Course Tools @ EH ✰ openvellum.ecollege.com/course.html?courseld=17762365&OpenVellumHMAC=57891f531d784362508555aa651bb397#10001 > e X Course Homearrow_forwarder| Portal x Edulastic O Science Spring Semester Exm bapp.edulastic.com/student/assessment/5f18467bc420a100086da 115/cla 28b60984e66etd62fac10/uta/6a9 Elementar. K! Kahoot R ReadWorks Play Quizizz! Newsela Assignm Commontty As ty Play no. Question 5/10 > NEXT BOOKMARK Water is boiled and steam is produced. This is a physical change because the water particles A. have turned into something new. B. are still water particles. C. have reacted with each other. D. have been destroyed. B.arrow_forwardd ← Chrome esc e 24% (92) ALE X Mathwa X G 102 cels X ☆ www-awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNsikr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHinMCnqLGstAPM iZtogOAPGJIWPnLIY... 17.4 Solubility and... 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... Y SOLUTION: The le... Math 115 W-S Fall.... File email d x Edit View History Bookmarks Profiles View 7. Q A Nancy X Z Explanation O KINETICS AND EQUILIBRIUM Using the Arrhenius equation to calculate k at one temperature... 2 W S The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E-29.0 kJ/mol. If the rate constant of this reaction is 2.8 x 10³ Ms at 320.0 °C, what will the rate constant be at 222.0 °C? -1 Round your answer to 2 significant digits. Check X A ALEKS X CIChego X H command # 3 E Tab Window Help D $ 4 C X R > F 5 (92) ALE X % 5 V I T G #tv Welcom X A ALEKS ^ 6 MacBook Pro B Y We 9 "/ & 7 H X U N You 8 J 0000015 1…arrow_forwardHgRhayfmbBs65spE8iXmXHI5RbYHS1kQ8hQVtMFFNR2t1ofvFgeoPQ0HvI6Tcpv7DtYEbxc Apps B Blackboard o Mail - Ava Schied.. O UAConnect 9 Biology Syllabus I Labflow - Courses Explore - HogSync O Packback t MyMercy - Login N Netflix e SafeAssign Selt O THERMODYNAMICS Using specific heat capacity to find heat Ava Calculate the energy required to heat 286.0 g of water from 49.2 °C to 58.7 °C. Assume the specific heat capacity of water under these conditions is 4.18 J-g K Round your answer to 2 significant digits. Explanation Check O 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use I. Privacy Accessibl MacBook Proarrow_forwardPosh x G hip hc x How x leks.com/alekscgi/x/isl.exe/1o_u-IgNslkr7j8P3jH-lvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInbMe2DNd12k5prizs0f2Tuz6MF2usVf2_c... Q 0 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po.... Ya SOLUTION: The le... Math 115 W-S Fall.... h. Gibbs Free E... 0,452 E CO 2 5.3 Enthalpies of... W ●ENTROPY AND FREE ENERGY Calculating dG from dH and dS S A chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 53.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions…arrow_forwardEdit View History Bookmarks People Tab Window Help O Questi x L 2021-0 x M [EXTE! X Dashb x O Launc O Launcix S Class eto.mheducation.com/ext/map/index.html?_con%3con&external_browser=0&launchUrl=https%253A%252F%252 20 Problem Set Saved attempts left Check my work Enter your answer in the provided box. Find AG for the following reaction, at 25°C, using AH and S values. f NHẠCI(s) → NH3(g) + HCI(g) kJ Standard Thermodynamic Values at 298 K Substance or Ion AH (kJ/mol) (J/mol K) HCI(g) HCI(aq) NH3(g) • NH3(aq) NH,CI(s) -92.3 186.79 55.06 -167.46 -45.9 193 -80.83 110 -314.4 94.6 ( Prey 6 of 15arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY