
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
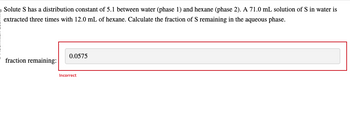
Transcribed Image Text:Solute S has a distribution constant of 5.1 between water (phase 1) and hexane (phase 2). A 71.0 mL solution of S in water is
extracted three times with 12.0 mL of hexane. Calculate the fraction of S remaining in the aqueous phase.
fraction remaining:
0.0575
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In an organic chemistry lab, some camphor crystals were synthesized but there were some traces of isoborneol also present in the crystals which lowered the melting point of the camphor crystals from 179 °C to 169 °C. Treating it as a solid-solid solution with isoborneol as the solute and camphor as the solvent, find the mass percentages of camphor and isoborneol in the solution. The freezing point depression constant for camphor is 40 °C kg mol1. The molecular weights of camphor and isoborneol are 152.2 g/mol and 154.3 g/mol respectively. O A. 96.14 % and 3.86 % respectively B. 3.80 % and 96.20 % respectively O C. 3.67 % and 96.33 % respectively O D. 96.29 % and 3.71 % respectively O E. 99.75 % and 0.25 % respectivelyarrow_forwardGiven the following mixture of two compounds 70.00 mL of X (MW =80.00 g/mol)(density 0.800 g/mL) and 820.00 mL of Y (73.00 g/mol))(density 1.056 g/mL). The freezing point of pure Y is 17.00 degrees C. The molal freezing constant is 3.091 degrees C/m. What is the freezing point of the solution, in degrees C?arrow_forwardSolute S has a distribution constant of 4.9 between water (phase 1) and hexane (phase 2). A 89.0 mL solution of S in water is extracted five times with 14.0 mL of hexane. Calculate the fraction of S remaining in the aqueous phase. fraction remaining:arrow_forward
- Solute S has a distribution constant of 5.1 between water (phase 1) and hexane (phase 2). A 71.0 mL solution of S in water is extracted three times with 12.0 mL of hexane. Calculate the fraction of S remaining in the aqueous phase. fraction remaining:arrow_forwardWhat is the concentration in molarity of an aqueous solution which contains 1.91% by mass ethylene glycol (MM = 62.07 g/mol)? The density of the solution is 1.04 g/mL. 1 4 7 +/- Tap here or pull up for additional resources 2.19 M 2 3 5 6 8 O XC с x 100arrow_forwardplease answer all wuestions with handwritten solutions and equationsarrow_forward
- A 2.00-g sample of a large biomolecule was dissolved in 15.0 g carbon tetrachloride.The boiling point of this solution was determined to be 77 .85°C. Calculate the molarmass of the biomolecule. For carbon tetrachloride, the boiling-point constant is 5.03°C∙ kg/mol, and the boiling point of pure carbon tetrachloride is 76.50°C.arrow_forwardA solution is prepared by adding 425g of AgNO3 to 0.150L of water at 40.0∘C. The solubility of AgNO3 at 40.0∘C is 311g100.mL. How many grams of AgNO3 are expected to precipitate if the temperature is dropped to 0.00∘C where the solubility of AgNO3 is only 122g100.mL? Give the answer with three significant figures.arrow_forwardHexane (P° = 152 torr) and cyclohexane (P° = 97.6 torr) form an ideal solution. At some solutionconcentration, the vapor above the solution is comprised of a 1:3 ratio of hexane:cyclohexanemolecules. What is the composition of the solution in mole percentages?arrow_forward
- Assuming 100% dissociation, calculate the freezing point (?f) and boiling point (?b) of 2.30 ? K3PO4(aq). Colligative constants can be found in the chempendix.arrow_forwardThe Henry’s law constant for the solubility of radon inwater at 300C is 9.57 x 10-6 M/mm Hg. Radon is presentwith other gases in a sample taken from an aquifer at 300C.Radon has a mole fraction of 2.7 x 10-6 in the gaseous mixture. The gaseous mixture is shaken with water at a totalpressure of 28 atm. Calculate the concentration of radonin the water. Express your answers using the following concentration units.(a) molarity(b) ppm (Assume that the water sample has a density of1.00 g/mL.)arrow_forwardThe vapor pressure of a solution containing 6.08 g of a nonvolatile, no electrolyte solute (C21H42O) in 60. 00 g of solvent A(MW=78 g / mol) is 684 torr at 20 °C. What is the vapor pressure of pure benzene at 20 °C Report answer to nearest whole numberarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY