
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
payelben
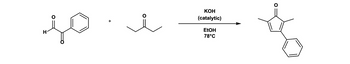
Transcribed Image Text:so
H
+
O
KOH
(catalytic)
EtOH
78°C
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- 3arrow_forwardO Select the strongest acid. O I— ④SH HOH H OH Iarrow_forwardB File Paste E + Home Cut E Copy 4 Page 1 of 1 Clipboard Format Painter Insert 0 words S Draw Design Layout References Calibri (Body) BIU U abe 11 À A Aa A A abe X₂ X² Font Type here to search P English (Philippines) Accessibility: Investigate Mailings 10 E•E•S• Review View Help HE e W Document1 - Word €¶ Paragraph 1-32. A bottle of concentrated aqueous sulfuric acid, labeled 98.0 wt% H₂SO4, has a concentration of 18.0 M. (a) How many milliliters of reagent should be diluted to 1.000 L to give 1.00 M H₂SO4? (b) Calculate the density of 98.0 wt% H₂SO4- S ■ Tell me what you want to do AaBbCcDc AaBbCcDc AaBbC AaBbCct AaB AaBbcct AaBbCcD Normal 1 No Spac... Heading 1 Subtitle Subtle Em... Heading 2 Title Styles Sign in 1:31 (Ctrl) - ENG S IX Share Find ab Replace Select Editing 8:09 pm 18/08/2022 ▸► + 228%arrow_forward
- What is the solubility of KCI at 80C 150 140 KI 130 120 110 100 NANO3 90 80 70 60 NH4CI KCI NacCl 50 40 30 20 KCIÓ3 10 Ce2(SO4)3 0 10 20 30 40 50 60 70 80 90 100 Temperature (°C) DELL Grams of solute per 100 g H,0 NH37 EONXarrow_forwardCl₂(g) + 2e Cr₂0 (aq) + 14 H(aq) + 6e¯ 02(g) +4H(aq) + 4e¯ MnO2(s) + 4H(aq) + 2e 103 (aq) +6H(aq) + 5e¯ VO₂ (aq) + 2H(aq) + e¯ Br₂() + 2e NO, (aq) + 4H(aq) + 3e¯ ClO2(g) + e Ag+(aq) + e 2 CT (aq) 1.36 2 Cr3+(aq) + 7 H₂O(1) 1.33 2 H₂O(1) 1.23 Mn2+(aq) + 2 H2O() 1.21 12(aq) + 3H2O(l) VO2+(aq) + H2O(l) 1.20 1.00 2 Br (aq) 1.09 NO(s) + 2 H2O(l) CIO₂(aq) 0.96 0.95 Ag(s) 0.80 0.77 Fe3+(aq) + e Fe2+(aq) A mixture of Fe3+, CIO₂, and Cr₂O,² was made, what reaction would be predicted, if any? a. Fe3+ + Cr2O7²- b. Cr2O + Clo²- c. Fe3+ Clo²- d. No reaction. -> -> Fe²+ + Cr³+. - Cr3+ + ClO2. Fe²+ + ClO2.arrow_forwardwhat is compresionarrow_forward
- Need help with homeworkarrow_forwardCl₂(g) + 2e Cr₂0 (aq) + 14 H(aq) + 6€¯ O2(g) +4 H(aq) + 4e¯ MnO2(s) + 4H(aq) + 2e¯ 10, (aq) +6H(aq) +5e¯ VO₂+ (aq) + 2H(aq) + e¯ Br₂(1) + 2e NO, (aq) +4 H(aq) + 3e¯ ClO2(g) + e 2 Cl(aq) Ag* (aq) + e Fe3+(aq) + e 2 Cr³+ (aq) + 7 H₂O(1) 1.36 1.33 2 H₂O(1) 1.23 Mn2+(aq) + 2 H₂O(1) 1.21 12(aq) + 3H2O(l) 1.20 VO²+(aq) + H₂O(1) 1.00 2 Br (aq) 1.09 NO(s) + 2 H2O(1) 0.96 CIO₂ (aq) 0.95 Ag(s) 0.80 Fe2+(aq) 0.77 What is the E° cell for the reaction Cr₂O²² + CIO₂ → Cr³+ + CIO₂? a. 2.28 V. b. -2.28 V. c. 0.38 V. d. -0.38 V.arrow_forwardChemistry 5’G-G-C-T-A-T-T-G-A-G•G-A-T-C-C-T-G-G-A-T-G-C-C3’3’C-C-G-A-T-A-A-C-T-C-C-T-A-G•G-A-C-C-T-A-C-G-G5’ a. Give the two DNA fragments generated by the enzyme, and then calculate the binding free energy for re-association of the two, resulting fragments.b. Calculate the equilibrium constant for the formation of the uncleaved complex at 25°C in 1M NaCl.c. Calculate the equilibrium constant for association of the ‘sticky ended DNA’ fragments at 25°C in 1M NaCl.d. Determine the concentration of ds DNA (in part c) after enzyme digestion, if the starting dsDNA concentration was 10-8M.arrow_forward
- aur X Macmilla X 2/ Course X 50 ~ T Sections X Submit Answer evo/index.html?deploymentid=5735112480241329813180832311&elSBN=9781305862883&id=1707786042&snapshot! References lancing Molecular Equations: This is group attempt 2 of 10 HOMEW X Use the References to access Important values if needed for this question. When the following molecular equation is balanced using the smallest possible Integer coefficients, the values of these coefficients are: Na (s) + H₂0 (1). NaOH (aq) + H₂ (8) ≡く □ □ A MindTap ㅁ Autosaved at 5:51 PM MacBook Air Gatoms to When the following molecular equation is balanced using the smallest possible Integer coefficients, the values of these coeffi Br₂(g) + Cl₂(g) →BrCl(g) X Domarrow_forwardObserve and collect the following data for following a sandwich recipe and making sandwiches! # of Complete Leftovers of Sandwiches Bread Leftovers of Cheese Which is limiting? (bread or cheese) O Scenario A B C Recipe 8 slices of bread 4 slices of cheese 8 slices of bread 3 slices of cheese 4 5 slices of bread 5 slices of cheese 3 2 1 2 For scenario B, cite evidence and reasoning for your "limiting" and "excess" ingredient assignments. neither cheese Which is in excess? (bread or cheese) neither bread 3 For scenario C, cite evidence and reasoning for your "limiting" and "excess" ingredient assignments.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY