
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:aur X
Macmilla X
2/
Course X
50 ~ T
Sections X
Submit Answer
evo/index.html?deploymentid=5735112480241329813180832311&elSBN=9781305862883&id=1707786042&snapshot!
References
lancing Molecular Equations: This is group attempt 2 of 10
HOMEW X
Use the References to access Important values if needed for this question.
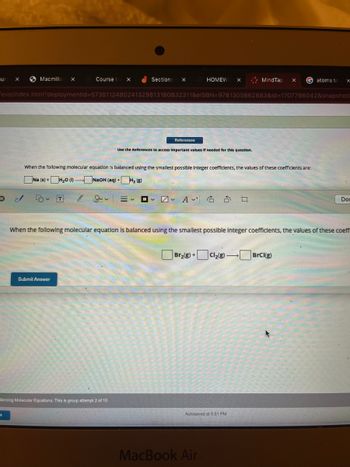
When the following molecular equation is balanced using the smallest possible Integer coefficients, the values of these coefficients are:
Na (s) + H₂0 (1).
NaOH (aq) + H₂ (8)
≡く □ □ A
MindTap
ㅁ
Autosaved at 5:51 PM
MacBook Air
Gatoms to
When the following molecular equation is balanced using the smallest possible Integer coefficients, the values of these coeffi
Br₂(g) + Cl₂(g) →BrCl(g)
X
Dom
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1010arrow_forwardi need the answer quicklyarrow_forwardName: Balancing Chemical Equations Balance the following equations using whichever method you prefer: 1) K (s) + MgBr2 (aq) → KBr (aq) + Mg (s) 2) CHSOH (I) + CO2 (g) + H20 (g) 3) P (s) + (a) 30 P20s (s) 4) CO: (g) + H2O (1) > O2 (g) + CH12O6 (s)arrow_forward
- Balance the following equation - If a coefficient is a one (1) PUT IT IN even though that would be optional if you were writing the equation yourself.arrow_forwardThe lead-acid storage battery is the oldest rechargeable battery in existence. It was invented in 1859 by French physician Gaston Plante and still retains application today, more than 150 years later. There are two reactions that take place during discharge of the lead-acid storage battery. In one step, sulfuric acid decomposes to form sulfur trioxide and water: H₂SO4(1)→ SO3(g) + H₂O(1) AH +113. kJ In another step, lead, lead(IV) oxide, and sulfur trioxide react to form lead(II) sulfate: Pb(s) + PbO₂(s) + 2SO3(g) 2 PbSO4(s) Calculate the net change in enthalpy for the formation of one mole of lead (II) sulfate from lead, lead(IV) oxide, and sulfuric acid from these reactions. Round your answer to the nearest kJ. kJ AH = -775. kJ Xarrow_forwardBalancing Chemical Equations Cu(s) O(g) → CuO(s) 28 Al+ H,SO. H + H0(1) → H(9) + 29 Si,H O, > SIO, HO Fe(s) H,O(g) → H:(g) Fe,O.(s) 30 NH, + H,O ASCI(aq) H;S(aq) → AS S(s) + HCI(aq) co, + 31. CuSO. 5H,0(s) → CuSO(s) + H;0(g) 32 BN + BF, + Fe Os(s) Hg) + Fe(S) +_H;O(1) 33. CaSO,+2H,0 + 50, → Caso, HSO. CaCO,(s) → CaO(s) + Co.(g) 34 C,H 0, CO, FeS(s) 35. C,H,0, 0. > CO, + • (s)3 HS(04) S(s) > HOH(I) + KS(aq) ZnI, > Nal Zn KOHaq) → 36 Na + 10. Na(1) + CI,(g) 37. HBr0, HBr > H,O Br. 11 Al(S) H,S0.(aq) + H(g) * Al,(SO,), 38 AI,C, + CH. NH,OHaq) + HOH(I) (NH.),PO,(aq) (beodH Ca(NO,),-3H,0 + LaC, Ca(NO,), La(OH CH 39 12. 13. CHIQ) CO.(9) H,0(1) 40. CH,NO, Cl, + CCI,NO, = HC Ca,(PO.), SiO, CaSiO, CO P 41. 14 AS) 0,9) + (s) o'iv CHE H,0(1) Al,C. + HO + AI(OH 42 15 CHdg) Co(g) * 43 Naf Cao + Caf NOOH 16 KINO, KNO, 44 AICI,> LIAIH. LICI 17 Coc Ca + Co, Caf,+ SiO, క్రచరి 18 CH + CO, + HO 45 HSO. 46 Casi, + SbCl, > CoCl 19. KSO KCI BaSO. TiO, B.C+ TiB CO 47 20 KOH + H,50. KSO. 48. NH, + NO HO 21…arrow_forward
- given the following equation 2 Co2O3(s) + 3 C(s) --> 4 Co(s) + 3 CO2(g) indicate the coefficient for elemental carbon 6 4 1 3 or 2arrow_forward7:25 ◄ Search 2- 3c₂²- 1 + Write a balanced chemical equation based on the following description: solid barium carbonate decomposes into solid barium oxide and carbon dioxide gas when heated BaCO3 (s) (s) Be O Question 5 of 20 Reset 2 3 4 5 6 7 8 B (1) Ox Ba Cb (g) C 个 Br Tap here or pull up for additional resources 5G 85 Ca Submit 9 11 (aq) O Cr • x H₂Oarrow_forwardBalance the following chemical equation (if necessary): C,H(g) + O,(g) –→ H̟O(g) + CO,(g) 04- 3. D2+ 3+ O4+ 1 4 6. 8. 9. 9. (s) (1) (g) (aq) CH0 CO, H̟O Reset • x H,O Delete 5arrow_forward
- Ca(OH)2 (s) + H₂S(g) →→→ CaS (s) + 2H₂O(l) In the reaction above, calcium hydroxide and hydrogen sulfide react to form calcium sulfide and water. If you combine 11 g of hydrogen sulfide with 15 g of calcium hydroxide: 1) Which reactant is limiting? [Select] 2) Calculate the number of moles of calcium sulfide produced if the percent yield is 100%. [Select] ✓ mol Casarrow_forwardO Mail O School Loop IT Help Synergy! O SFUSD_bookmarks G Image result for car. YouTube Question 4 of 40 Balance the following chemical equation (if necessary): NasPO:(aq) + NiCl2(aq) → Nis(PO4)2(s) + NaCI(aq) 04-03-0²-|D- 03 4+ 2+ 4 8 0. O O60, Os O, Do 1 (s) (1) (g) (aq) Nis(PO:)2 NaCI NiCl Reset •x H:O Delete 91 7. 3. 2.arrow_forward"Smelling salts," which are used to revive someone who has fainted, typically contain ammonium carbonate, (NH4)2CO3 . Ammonium carbonate decomposes readily to form ammonia, carbon dioxide, and water. The strong odor of ammonia usually restores consciousness in the person who has tainted. The unbalanced equation is: ( NH4 )2 CO3( s)→ NH3(g ) + CO2( g) + H2O(g ) calculate the mass of ammonia gas that is produced if 1.25 g of ammonium carbonate decomposes completely.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY