
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
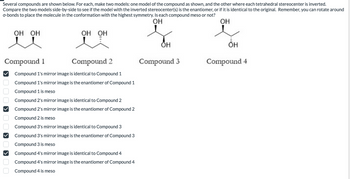
Transcribed Image Text:Several compounds are shown below. For each, make two models: one model of the compound as shown, and the other where each tetrahedral stereocenter is inverted.
Compare the two models side-by-side to see if the model with the inverted stereocenter(s) is the enantiomer, or if it is identical to the original. Remember, you can rotate around
o-bonds to place the molecule in the conformation with the highest symmetry. Is each compound meso or not?
OH
OH
OH OH
DOKOOK
OH OH
Compound 1
Compound 2
Compound 1's mirror image is identical to Compound 1
Compound 1's mirror image is the enantiomer of Compound 1
Compound 1 is meso
Compound 2's mirror image is identical to Compound 2
Compound 2's mirror image is the enantiomer of Compound 2
Compound 2 is meso
Compound 3's mirror image is identical to Compound 3
Compound 3's mirror image is the enantiomer of Compound 3
Compound 3 is meso
Compound 4's mirror image is identical to Compound 4
Compound 4's mirror image is the enantiomer of Compound 4
Compound 4 is meso
OH
Compound 3
ÕH
Compound 4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Determine whether the following statements are true or false. Completely fill in the circle in front of your chosen answer. (a) A compound with a plane of symmetry must be achiral. (b) A compound with two or more stereocenters must be chiral. (a) O True O False True O Falsearrow_forwardPlease don't provide handwritten solution... Please provide an explanationarrow_forward(R)-Lyrica has an optical rotation of -128 degrees in acetone solution and it's crystals have a melting point of +184 degrees celsius. It's enantiomer, (S)-Lyrica, would have an optical rotation of ______ and a melting point of _____?arrow_forward
- Draw a structural formula of the SR configuration of the compound shown below. HO CH3 S -NHCH3 R H3C ephedrine • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • Include H atoms at chiral centers only. • If a group is achiral, do not use wedged or hashed bonds on it. * #[ ] در ?arrow_forwardPlease solve correctly asaparrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY