
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please help with the highlighted problem attached
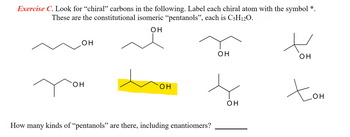
Transcribed Image Text:Exercise C. Look for “chiral” carbons in the following. Label each chiral atom with the symbol *.
These are the constitutional isomeric “pentanols”, each is C5H12O.
он
ОН
он
он
How many kinds of "pentanols” are there, including enantiomers?
он
он
н
он
Хон
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hello! I am having a hard time with this problem. I am unsure of what to do and the questions keep giving me feedback that the formatting is wrong. I put in an answer below of what I thought was correct, but is not. Can you please explain how to do this problem? Anything is appreciated! Thanks so much!arrow_forwardFile Response Question. Draw the molecular structure of a phospholipid, showing a glycerol backbone and associated bonding. Briefly explain why this molecule could be considered Amphipathic (write your answer under your drawing). Word count: 50 words (in total). Attach File Browse My Compuer Browse Coment Collectionarrow_forwardb. متنا للہ OHarrow_forward
- draw an arrow pushing mechanism for this examplearrow_forwardHow many grams of N2 are needed to completely (stoichiometrically) react with 222 g of Br2 ? N2 + 3 Br2 2 NB13 + 44.0 kJ The molar mass of Br2 is g and the molar mass of N2 is mol Br2 (2.22 x 102 g Br2)(- mol Br2 g Br2 mol N2 mol Br2 (-- -) = mol N2 mol Br2 g N2 mol N2 (-- -) = |v g N2 mol N2 а. 1 b. 2 С. 3 d. 4 е. 5 f. 253.719 g. 3.38 х 100 h. 3.26 x 103 i. 44.0 j. 1.33 x 102 k. 9.77 x 103 I. 4.44 x 102 m. 6.66 x 10² n. 500.0 o. 556 р. 90.0 q. 3.2852 r. 159.81 s. 28.013 t. 1.750 u. 0.8750 v. 13.0 w. 0.46306 х. 1.3892 у. 24.5 z. 38.5 аа. 2.190 bb. evolved Cc. absorbed dd. exothermic ee. endothermicarrow_forwardcheck the imagearrow_forward
- IDO BEGINNING ON MONDAY 8TH FEBRUARY, 2021. DEASE COMPLETE YOUR TUTORIAL SHEET FOR DISCUSSIONS IN YOUR TUTORIAL SESSIONS NB: Tutorial sheets will be handed out weekly, each one containing a few questions covering material from the previous week's lectures. The material on the sheets will be discussed at the tutorial sessions. Please revise your lecture notes on a weekly basis, do the necessary background reading and attempt the tutorial sheets. 1) a) Draw curved arrow(s) to show how aniline is converted to its resonance structure A and how resonance structure A is converted to resonance structure B. Draw the resonance structure C. > B C Aniline b) The following resonance structures are used to describe the molecule diazomethane. It is a bright yellow, volatile, toxic, explosive and useful gas. H,C=N=N: + H,C-N=N: Indicate the presence of any formal charges on these resonance structures by placing + and/or - signs above the appropriate atoms. Show all such charges in both structures.…arrow_forwardhow does the ring open? can you add more steps?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY