
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
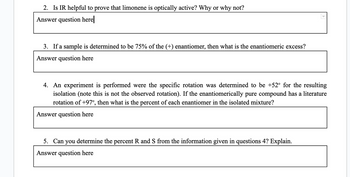
Transcribed Image Text:2. Is IR helpful to prove that limonene is optically active? Why or why not?
Answer question here
3. If a sample is determined to be 75% of the (+) enantiomer, then what is the enantiomeric excess?
Answer question here
4. An experiment is performed were the specific rotation was determined to be +52° for the resulting
isolation (note this is not the observed rotation). If the enantiomerically pure compound has a literature
rotation of +97°, then what is the percent of each enantiomer in the isolated mixture?
Answer question here
5. Can you determine the percent R and S from the information given in questions 4? Explain.
Answer question here
Expert Solution
arrow_forward
Step 1: Introduction of compound
Limonene is a monoterpene compound.
Note ~ Since you have posted multiple questions, we will provide the solution
only to the first question as per our Q&A guidelines. Please repost the
remaining questions separately.”
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw all stereoisomers of Limonene using filled and dashes wedgesarrow_forwardces to access question. Draw a structural formula for one of the 5 constitutional isomers of the unbranched alkane C7H16 in which the longest carbon chain has 5 atoms. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. CH4 {n [ ? ChemDoodleⓇarrow_forwardWhich molecule does not contain a conjugated system?arrow_forward
- Recitation Discussion Problem: USE MOLECULAR MODELS and bring them to recitation. Molecule: 2-methylpentane 1. Draw in bond-line notation. Number all carbons in the pentane backbone. Identify carbons #2 -3. Circle these on your bond-line structure. 2. Draw the Newman projections for all 60° rotations looking down carbons #2-3- starting with the eclipsed conformation. Make sure to keep the front carbon static only rotate the back carbon. Use models. You should draw seven Newman projections in total, 4 eclipsed, 3 staggered. The first and last Newman projections (0° and 360°) are identical. See Karty Chapter 4 for examples. 3. Identify the major interactions occurring in each conformer (which bonds are eclipsed; which have gauche interactions, etc.) and RANK associated energy of eclipsed in terms of size of group. Greater energies are involved for larger eclipsed groups. See Karty Chapter 4 energy diagram examples. 4. Plot these conformations on an Energy Diagram (similar to Karty Figure…arrow_forwardDraw and name two constitutional isomers that have only one stereocenter and are named “ ...ethyl ...hexane”.arrow_forwardDraw and name a constitutional isomer that has two stereocenters and is named a“....tetramethylhexane”.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY