
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
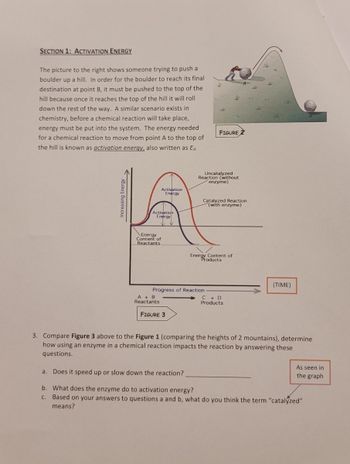
Transcribed Image Text:SECTION 1: ACTIVATION ENERGY
The picture to the right shows someone trying to push a
boulder up a hill. In order for the boulder to reach its final
destination at point B, it must be pushed to the top of the
hill because once it reaches the top of the hill it will roll
down the rest of the way. A similar scenario exists in
chemistry, before a chemical reaction will take place,
energy must be put into the system. The energy needed
for a chemical reaction to move from point A to the top of
the hill is known as activation energy, also written as Ea.
Increasing Energy.
Activation
Energy
Activation
Energy
Energy
Content of
Reactants
A + B
Reactants
FIGURE 3
J
Progress of Reaction.
t
$
Uncatalyzed
Reaction (without
enzyme)
Energy Content of
Products
V
FIGURE 2
Catalyzed Reaction
(with enzyme)
C + D
Products
A
م
اسکار
(TIME)
3. Compare Figure 3 above to the Figure 1 (comparing the heights of 2 mountains), determine
how using an enzyme in a chemical reaction impacts the reaction by answering these
questions.
As seen in
the graph
a. Does it speed up or slow down the reaction?
b. What does the enzyme do to activation energy?
c. Based on your answers to questions a and b, what do you think the term "catalyzed"
means?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Can someone give me an understanding of Products and Reactants? The first image is from the the first chapter where it was introduced under the subject of life, chemistry and water. The second image is on another chapter I am speaks under the subject of Energy, Enzymes and Biological reactions. I can't understand it without the root understanding and application of Products and Reactants.arrow_forwardSECTION 4: FACTORS AFFECTING ENZYME RATE You now know that enzymes have a very specific shape and they must retain their shape in order to function properly. There are many factors that can change the shape of an enzyme and/or change the rate at which it works. Concentration of enzyme or substrate, temperature and pH are just a few factors that have the potential to change the rate of a chemical reaction. 16. With the knowledge that a lot of enzymes work within the human body, hypothesize what temperature and pH might be optimal for enzymes to work properly. Analyze the experiment below to determine the effects of temperature and pH on enzymatic activity. H₂O2 is produced as a byproduct of many metabolic processes, but it is toxic to most living organisms. Many organisms are capable of enzymatically destroying the H₂O2 before it can do much damage. H₂O₂ can be converted to oxygen and water, as follows: 2 H₂O2 → 2 H₂O + O₂ Although this reaction occurs spontaneously, enzymes increase…arrow_forwardI need the answer as soon as possiblearrow_forward
- MC: Which of the following are true of an enzyme? An enzyme is never needed for an exothermic reaction An enzyme works by minimizing the AG An enzyme is never consumed in a reaction An enzyme is not always a proteinarrow_forwardChemical reactions in our body are generally one of three types, in this type of reaction a molecule is generally broken down and energy is released. anabolic hydrogen catabolic exchangearrow_forwardWhich statement is correct for the reaction shown? Glucose ATP ADP Glucose 6-phosphate Glucose and ATP are reactants, and a phosphate is transferred from glucose to ATP. Glucose and ADP are reactants, and a phosphate is transferred from glucose to ADP. Glucose and ATP are reactants, and a phosphate is transferred from ATP to glucose. Glucose and ADP are reactants, and a phosphate is transferred from ADP to glucose.arrow_forward
- Energy can be classified into both kinetic energy and potential energy. Which image best illustrates kinetic energy? A baseball in a pitcher's hand. A puck slides past a goalie. A book balances on someone's head. A boulder sits on a cliff. None of these. a. b. C. d. e. a d C barrow_forwardUnder which of the following conditions could a biochemical reaction inside a cell have a positive enthalpy change and still occur spontaneously? (Recall that AG = AH – TAS) O When the reaction is in rapid equilibrium. O When there is a large increase in entropy. O When the AG is positive. O At an enzyme active site under Vmay conditions. O None of the above.arrow_forwardWhy does the product of each chemical reaction contain less energy than the starting substrate? Fuel substrates gain energy during metabolism. The product of the reaction contains more bonds. Chemical reactions are always anabolic. The product of the reaction contains fewer bonds.arrow_forward
- Enzymes are biological catalysts that enhance the rate of a reaction by: stabilizing the transition state. decreasing the amount of free energy released. increasing the activation energy. increasing the amount of free energy released. O increasing the energy in the transition state.arrow_forwardWhich of the following is a description of an example of the second law of thermodynamics? Some chemical energy in glucose transforms to chemical energy in ATP. O The kinetic energy of wind turns the blades of a wind turbine. The chemical energy in gasoline is transformed to kinetic energy to drive a car. The mechanical energy of flowing water turns a turbine. Some chemical energy in gasoline is transformed to heat while driving a car.arrow_forwardEnzymes are: [Select all that apply.] O mostly proteins. molecules that are a main source of energy for most cells. molecules that catalyze (speed up) other chemical reactions. O molecules that have specific shape that bind specific molecules. O waste products from most cellular chemical reactions. O used up in the chemical reactions that they catalyze.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education