
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
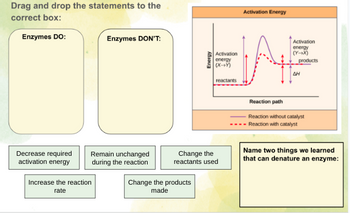
Transcribed Image Text:Drag and drop the statements to the
correct box:
Enzymes DO:
Decrease required
activation energy
Enzymes DON'T:
Remain unchanged
during the reaction
Increase the reaction
rate
Energy
Change the products
made
Activation
energy
(X+Y)
reactants
Change the
reactants used
Activation Energy
A
Activation
energy
(Y→X)
products
ΔΗ
Reaction path
Reaction without catalyst
Reaction with catalyst
Name two things we learned
that can denature an enzyme:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- Enzymes are important biological catalysts because they: Lower the activation energy of a biochemical reaction Lower the entropy of a biochemical reaction Increase the free energy of a biochemical reaction Supply the energy to initiate a biochemical reactionarrow_forwardFigure #1 and #2 show the results collected from a fun enzyme activity lab that did NOT involve the liver;) Answer the questions that follow. REACTION RATE (mg/sec) -1 m O 0 1 2 3 4 5 6 Rate of Reaction Enzyme A Rate ofreaction pH Figure #1 pH and enzyme reaction rate 0 10 7 8 9 10 11 12 13 14 Enzyme B Temperature 35 40 Enzymes A and B Figure # 2 The effect of temperature (°C) on enzyme activity a. Looking at these results, could these enzymes (A and B) be found somewhere in the human body? If so, where? Explain your answer, and be sure to discuss both factors (pH and temperature) as they relate to enzyme activity/rate of reaction.arrow_forwardRefer to Model 10.1 and answer the question that follows What is free energy? What is its symbol?arrow_forward
- Describe how enzymes speed up chemical reactions (both energetically and physically!), and how they affect the energy and equilibrium of a reaction. Describe 6 different physical and chemical factors that can regulate enzyme activity.arrow_forwardTrue/False Question: Enzymes do not shift the equilibrium of the reactions that they catalyze. O True O ralsearrow_forwardMatch the letters with the following uncatalyzed products free energy progress of reaction catalyzed reaction activation energy without an enzyme net change in energy between reactants and products reactants activation energy with an energyarrow_forward
- Using the table below can you please help me answer the following. Based on your results, what can you conclude about the effect of enzyme concentration on reaction rate? Explainarrow_forwardIn the diagram, notice the y-coordinate (energy) of A + B; also notice how C is at a lower y-coordinate. Their difference is the amount of energy during the exothermic reaction. A+B Activation Energy Reaction Progression Energyarrow_forwardWhich one of the following statements is completely TRUE? O When AG > 0, the reaction is BOTH product-favored (spontaneous) AND endergonic. When AG 0, the reaction is BOTH reactant-favored (nonspontaneous) AND endergonic. When AG > 0, the reaction is BOTH product-favored (spontaneous) AND exergonic. When AG > 0, the reaction is BOTH reactant-favored (nonspontaneous) AND exergonic. When AG < 0, the reaction is BOTH reactant-favored (nonspontaneous) AND exergonic.arrow_forward
- Consider the following free energy diagram for an uncatalyzed and enzyme-catalyzed reaction. Select all the statements that are true. Without enzyme With enzyme A+B Time AB Oa. The reaction is now spontaneous due to the addition of enzyme b. The rate of the enzyme catalyzed reaction is faster than the uncatalyzed reaction O C. The reaction is exergonic O d. The change in free energy for the reaction is greater in the catalyzed reaction, compared to the uncatalyzed reaction e. The enzyme stabilizes the transition state for the reaction Released Energy pesarrow_forwardWhy can’t enzyme kinetics prove that a particular enzyme mechanism is correct?arrow_forwardWhich of the following is NOT a true statement about the diagram below? Intermediate A Intermediate B End-product Pathway operates 2019 Pearson Education, Inc. O O O O Substrate Enzyme 1- Allosteric site -Enzyme 2 Enzyme 3- Pathway shuts down Bound end-product Feedback Inhibition The end-product None of the other four answers (all are true statements) Each enzyme is specific for its substrate The product of each enzyme reaction becomes a substrate for the next enzyme The diagram shows a metabolic pathway serves as a competitive inhibitor of the substrate on Enzyme 1arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON