
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Search...
nvellum.ecollege.com/course.html?courseld3D15432274&HepID=2b3e48e6520860bfd5591538a4a5a27b#10001
Ae. AOL Video - Serving the best vi.
ine Sh... TripAdvisor
<MC 13 on Temperature, Changes in Energy, and Gases
28 of 40
Item 28
Review | Constants | Periodic Table
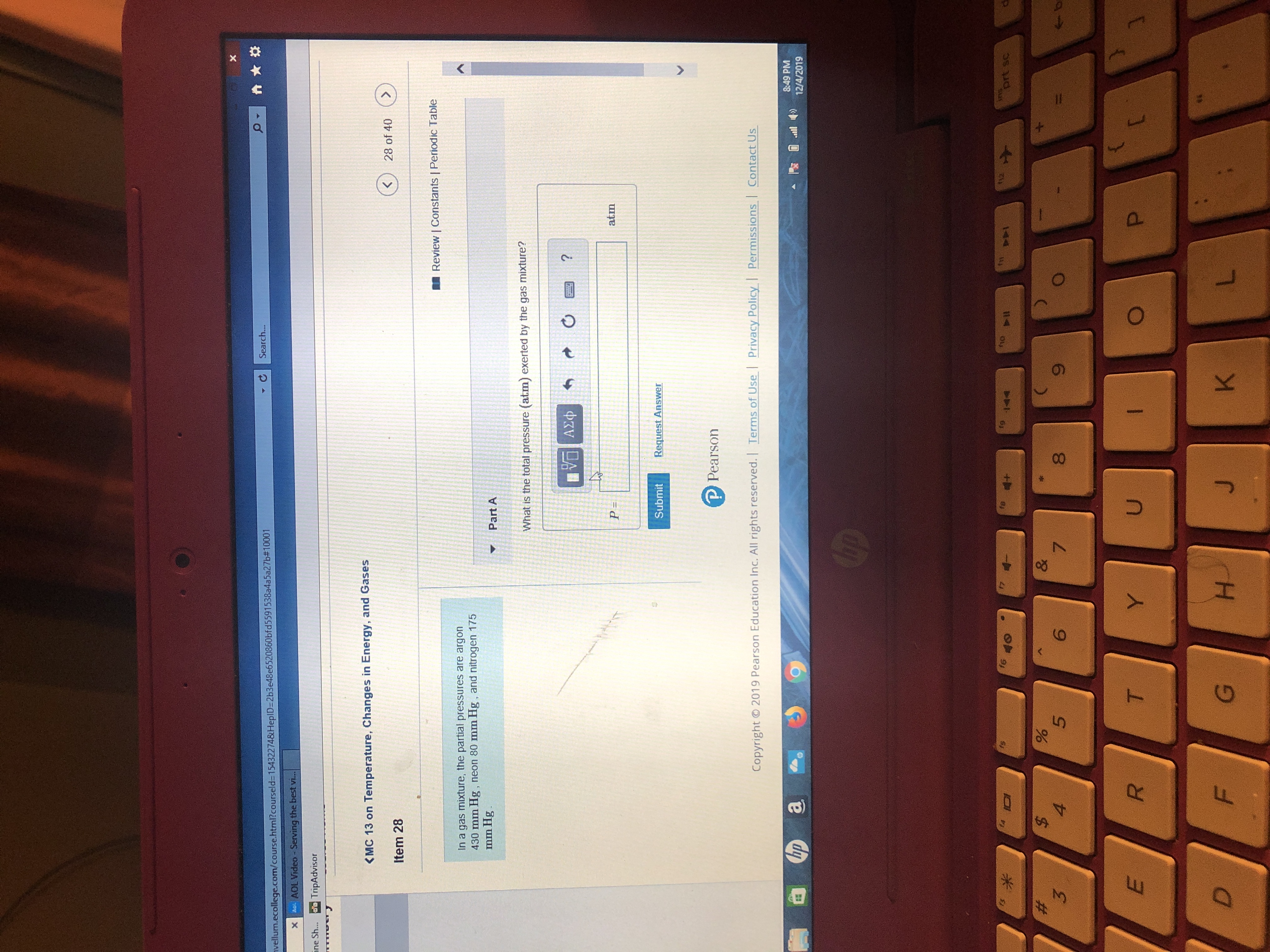
In a gas mixture, the partial pressures are argon
430 mm Hg, neon 80 mm Hg, and nitrogen 175
mm Hg.
Part A
What is the total pressure (atm) exerted by the gas mixture?
Vα ΑΣφ
atm
Request Answer
Submit
P Pearson
Contact Us
Permissions
Privacy Policy
Terms of Use
Copyright © 2019 Pearson Education Inc. All rights reserved.
8:49 PM
12/4/2019
ip
STAS
prt sc
12
fg
f6
f4
144
%23
3
%$4
&
8.
7.
T.
P.
Y.
D.
H.
F.
%24
LI
%23
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Please answer question 2arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardStandard temperature and pressure (STP) are considered to be 273 K and 1.0 bar. Predict which of the following changes will cause the volume of the balloon to increase or decrease assuming that the temperature and the gas filling the balloon remain unchanged. Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Help Balloon filled with helium at STP floats into the atmosphere where the pressure is 0.5 bar. Balloon filled with helium at STP floats Balloon filled with helium under water into air where the pressure equals 1 bar. at 1.15 bar is released and floats to the surface, which is at STP. Balloon filled with helium at STP is submerged under water where the pressure is 1.25 bar. Volume increases Volume decreases Volume is unchangedarrow_forward
- colle com/course.html?courseld3D169856748OpenVellumHMAC=Da46387336edc299b16e49c591bf9ad36#10001 For Practice 10.5 - Enhanced - with Feedback I Review | Constants | Periodic Table An 8.45 L tire contains 0.532 mol of gas at a temperature of 320 K. You may want to reference (Pages 425 - 430) Section 10.5 while completing this problem. Part A What is the pressure (in atm) of the gas in the tire? Express the pressure to three significant figures and include the appropriate units. HA ? Value Units Submit Request Answer Next > Provide Feedback P Pearson 11:17 AM A a O (x a 12/10/2021 75°F Partly sunny arch BHJC04Z022B01 E7 O010ahotmai) com 远arrow_forwardAmmonia gas is produced from nitrogen gas and hydrogen gas. A. Write a balanced chemical equation for this reaction. B. At STP, what mass (in grams) of nitrogen is needed for the complete reaction of 15.1 L of hydrogen? Edit View Insert Format Tools Table 12pt v Paragraph v B IUAV2V ぴく O words レ7 …arrow_forward← = Account Balance C O STATES OF MATTER Using the ideal equation of state volume: L Explanation 62°F Sunny ^? 7 Q A x Schedule Builder A reaction at 14.0 °C evolves 523. mmol of carbon dioxide gas. Calculate the volume of carbon dioxide gas that is collected. You can assume the pressure in the room is exactly 1 atm. Round your answer to 3 significant digits. Z https://www-awu.aleks.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-IBMBkpcnaFuOF7Uj... Check 2 W S X alt # 3 * E D 14 $ C X O -boleh- 4 X Give to Queensborough X R F % 3 do 5 to 4- T A 6 G 4+ B & 27 hp H Announcements-2022 F XA ALEKS-Chisom Obidiegw X A B ( 67 144 N * 8 D-II ( ـــالــــال الـالتـال M ✔ to 9 Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acces Ⓒ 40 11:27 11/3/20 K D-D-I > O ▬▬▬▬▬▬▬▬ L W 1- P S alt + [. 0/5 ? "4 + prt sc 1 ♡ C pause backspacearrow_forward
- A gas mixture composed of helium and argon has a density of 0.765 g/Lat a 736 mmHgand 298 K. Part A What is the composition of the mixture by volume?Express your answer with the appropriate units. VHeVtotal V H e V t o t a l , VArVtotal = nothing% SubmitRequest AnswerProvide FeedbackNextarrow_forwardAn airplane cabin is pressurized to 640 mmHg. Calculate the cabin pressure in kilopascals (kPa). 640 mmHg TOOLS x10 about us careers privacy policy terms of use contact us help 1.docx ZoomDay20sp21.pdf Hypothesis Test..pdf Quiz #19 (Online)..pdf 87,253 W MacBook Air F10 F9 DOO D00 F4 F7 * 8 5arrow_forward100 nd X SAsealed balloon is filled with S A sealed balloon is filled with x G celsius to kelvin - Google Sear X + /takeCovalentActivity.do?locator=Dassignment-take VolState e My Account The Common Appli... e elearn 9 YouScience The College Board .. T Login canvas [Review Topica] [References] Use the References to access important values if needed for this question. A sample of fluorine gas has a density of gL at a pressure of 0.580 atm and a temperature of 35 °C Assume ideal behavior. Submit Answer Retry Entire Group 9 more group atternpts lemainingarrow_forward
- Number 2arrow_forwardA rigid cylinder of gas shows a pressure of 600 torr at 215 K. The cylinder is moved to a new storage site where the pressure within the cylinder is now measuring 750 torr. What is the new temperature? Show your work. Answer in a complete sentence. H. = = = BIUS Enter your answer here 300 words left Page 13 of ?2arrow_forward-bit-6: Mastery ots pts pts pts 3 pts 2req M pts 2req pts 2req 1 pts 1 pts 2req (M) ... east.cengagenow.com [Review Topics] [References] Use the References to access important values if needed for this question. A sample of helium gas collected at a pressure of 0.885 atm and a temperature of 297 K is found to occupy a volume of 634 milliliters. How many moles of He gas are in the sample? mol Submit Answer Retry Entire Group 9 more group attempts remaining 53% + 88arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY