
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
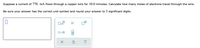
Transcribed Image Text:Suppose a current of 770. mà flows through a copper wire for 10.0 minutes. Calculate how many moles of electrons travel through the wire.
Be sure your answer has the correct unit symbol and round your answer to 3 significant digits.
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A current of 3.68 A is passed through a Sn(NO3)2 solution. How long, in hours, would this current have to be applied to plate out 5.40 g of tin?arrow_forwardA current of 6.6 A is passed through a solution of AgNO3 to electroplate it onto a surface. Calculate the mass, in grams, of pure silver deposited on the surface after 77 seconds.arrow_forwardA current of 5.55 A is passed through a Cu(NO3)₂ solution for 1.90 h. How much copper is plated out of the solution? mass of copper: garrow_forward
- If a current of 40.0x10-6 A is drawn from a solar cell for six months, how many faradays are involved?arrow_forward41. What mass of gold is produced when 16.8 AA of current are passed through a gold solution for 10.0 minmin ? Express your answer with the appropriate units.arrow_forwardWhat mass of aluminum metal can be produced per hour in the electrolysis of a molten aluminum salt by a current of 30 A ? Express your answer using two significant figures.arrow_forward
- Suppose 830. mmol of electrons must be transported from one side of an electrochemical cell to another in 231. seconds. Calculate the size of electric current that must flow. Be sure your answer has the correct unit symbol and the correct number of significant digits.arrow_forwardIn the Hall-Heroult process, a large electric current is passed through a solution of aluminum oxide (Al,0,) dissolved in molten cryolite (Naz AIF, resulting in the reduction of the Al,0, to pure aluminum. Suppose a current of 8300. A is passed through a Hall-Heroult cell for 29.0 seconds. Calculate the mass of pure aluminum produced. Be sure your answer has a unit symbol and the correct number of significant digits. ロロ のarrow_forwardHow many grams of magnesium metal will be deposited from a solution that contains Mg2+ ions if a current of 0.523 A is applied for 49.3 minutes. gramsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY