
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
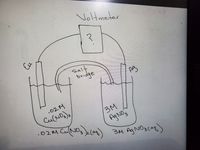
Transcribed Image Text:Voltmeter
Salt
bridge
Ag
.02M
Cu(Ni
3M
AgNOS
02MC
3M Aq NO3 cag
)

Transcribed Image Text:Using the attached Galvanic Cell under Non Standard condition, calculate Ecell.
Galvanic Cell Non standard Condition.pdf 1249 KB
A. 0.52V
O B. 0.80V
O C. 1.17V
O D. 2.17V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A standard galvanic cell is constructed so that the overall cell reaction is 2A13++(aq)+3M(s)3M2+(aq)+2A1(s) Where M is an unknown metal. If G = 411 kJ for the overall cell reaction, identify the metal used to construct the standard cell.arrow_forwardElectrochemical Cells II Consider this cell running under standard conditions: Ni(s)Ni2(aq)Cu+(aq)Cu(s) a Is this cell a voltaic or an electrolytic cell? How do you know? b Does current flow in this cell spontaneously? c What is the maximum cell potential for this cell? d Say the cell is connected to a voltmeter. Describe what you might see for an initial voltage and what voltage changes, if any, you would observe as time went by. e What is the free energy of this cell when it is first constructed? f Does the free energy of the cell change over time as the cell runs? If so, how does it change?arrow_forwardThe mass of three different metal electrodes, each from a different galvanic cell, were determined before and after the current generated by the oxidation-reduction reaction in each cell was allowed to flow for a few minutes. The first metal electrode, given the label A, was found to have increased in mass; the second metal electrode, given the label B, did not change in mass; and the third metal electrode, given the label C, was found to have lost mass. Make an educated guess as to which electrodes were active and which were inert electrodes, and which were anode(s) and which were the cathode(s).arrow_forward
- a Calculate G for the following cell reaction: Tl(s)Tl+(aq)Pb2+(aq)Pb(s) The Gf for Tl+(aq) is 32.4 kJ/mol. b From G, calculate the standard cell potential for the cell reaction and from this, determine the standard potential for Tl2+(aq)+eTl(s).arrow_forwardFor each reaction listed, determine its standard cell potential at 25 C and whether the reaction is spontaneous at standard conditions. (a) Mn(s)+Ni2+(aq)Mn2+(aq)+Ni(s) (b) 3Cu2+(aq)+2Al(s)2Al3+(aq)+3Cu(s) (c) Na(s)+LiNO3(aq)NaNO3(aq)+Li(s) (d) Ca(NO3)2(aq)+Ba(s)Ba(NO3)2(aq)+Ca(s)arrow_forwardConsider a galvanic cell based on the following half-reactions: a. What is the expected cell potential with all components in their standard states? b. What is the oxidizing agent in the overall cell reaction? c. What substances make up the anode compartment? d. In the standard cell, in which direction do the electrons flow? e. How many electrons are transferred per unit of cell reaction? f. If this cell is set up at 25C with [Fe2+] = 2.00 104 M and [La3+] = 3.00 103 M, what is the expected cell potential?arrow_forward
- An aqueous solution of an unknown salt of gold is electrolyzed by a current of 2.75 amps for 3.39 hours. The electroplating is carried out with an efficiency of 93.0%, resulting in a deposit of 21.221 g of gold. a How many faradays are required to deposit the gold? b What is the charge on the gold ions (based on your calculations)?arrow_forwardA galvanic cell is constructed in which the overall reactionis Cr2O72(aq)+14H2O+(aq)+6I(aq)2Cr3+(aq)+3I2(s)+21H2O(l) Calculate E for this cell. At pH 0, with [Cr2O72]=1.5M and [I]=0.40M, the cell potential is found to equal 0.87 V. Calculatethe concentration of Cr3+(aq) in the cell.arrow_forwardThe standard potential of the cell reaction Ag+(aq)+Eu2+(aq)Ag(s)+Eu3+(aq) is E = +1.23 V. Use the tabulated standard potential of the silver half-reaction to find the standard reduction potential for the europium half-reaction.arrow_forward
- For each of the reactions, calculate E from the table of standard potentials, and state whether the reaction is spontaneous as written or spontaneous in the reverse direction under standard conditions. (a) Cu2+(aq)+Ni(s)Cu(s)+Ni2+(aq) (b) 2Ag(s)+Cl2(g)2AgCl(s) (c) Cl2(g)+2I(aq)2Cl(aq)+I2(s)arrow_forwardDetermine the overall reaction and its standard cell potential at 25 C for the reaction involving the galvanic cell in which cadmium metal is oxidized to 1 M cadmium(II) ion and a half—cell consisting of an aluminum electrode in 1 M aluminum nitrate solution. 15 the reaction spontaneous at standard conditions?arrow_forwardDetermine the overall reaction and its standard cell potential at 25 C for these reactions. Is the reaction spontaneous at standard conditions? Assume the standard reduction for Br2(l) is the same as for Br2(aq).. Pt(s)|H2(g)|H+(aq)Br2(aq),Br(aq)|Pt(s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning