
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN: 9781305079250
Author: Mark S. Cracolice, Ed Peters
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:Review Homework REQUIRED (NOT Pie Progress)
Question 16 of 46 (2 points) | Question Attempt: 1 of Unlimited
✓ 14
15
16
17
18
19
20
21
✓ 22
✓ 23
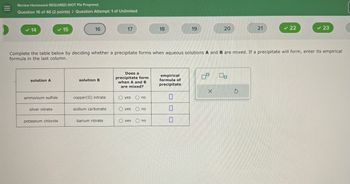
Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical
formula in the last column.
solution A
solution B
Does a
precipitate form
when A and B
are mixed?
empirical
formula of
precipitate
Do
ammonium sulfide
copper(II) nitrate
Oyes no
☐
silver nitrate
sodium carbonate
O yes no
potassium chloride
barium nitrate
O yes no
☐
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- please don't provide hand written soluton....arrow_forwardSolid lead(II) nitrite is slowly added to 125 mL of a 0.220 M ammonium iodide solution until the concentration of lead ion is 0.0272 M. The percent of iodide ion remaining in solution is %. Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. A solution contains 1.24x10-² M silver nitrate and 9.41x10-³ M calcium acetate. Solid ammonium sulfite is added slowly to this mixture. What is the concentration of silver ion when calcium ion begins to precipitate? [Ag+] = M Submit Answer Retry Entire Group 2 more group attempts remainingarrow_forward
- 1. Molarity of the NaOH solution 0.238 mol/L Trial 1 Trial 2 Trial 2. Volume of H;PO4 added to flask 22.0 mL 22.0 mL 22.0 mL 3. Initial NaOH volume _0.25_ mL _0.75_ mL 0.55 mL 4. Final NaOH volume 17.60_ mL 18.20_ mL _17.90_ mL 5. NAOH volume used for titration to reach green end point mL mL mL 6. NaOH volume used for titration 7. Moles of NaOH used for titration mol mel mel 8. Moles of H;PO4 that reacted mol mel mol 9. Volume of H;PO4 added to flask L 10. Molarity of H3PO4 mol/L mol/L mol/Larrow_forwardNeeded urgently... do it asap... STRICTLY HANDWRITTEN ONLYarrow_forward2- What is the concentration of Cro, required (molarity) in a solution that is 0.002 M in Ba2* in order to cause a precipitate to begin to form? The Ksp for BaCrO4 is 8.16x10-". Report your answer to the tenths place and enter your answer using scientific notation.arrow_forward
- Number 1arrow_forwardB. Molar Solubility of Calcium Hydroxide in the Presence of a Common Ion Trial 1 Trial 2 Trial 3 OM 1. Volume of saturated Ca(OH), with added CaCl, solution (mL) 25.0 25.0 25.0 0.05956 16.80 32.9 2. Concentration of standardized HCI solution (moVL) 3. Buret reading, initial (mL) O.00 33.5 4. Buret reading, final (mL) 10.80 48 5. Volume of HCI added (mL) 6. Moles of HCI added (mol) 7. Moles of OH¯ in saturated solution (mol) 8. (OH), equilibrium (moVL) 9. Molar solubility of Ca(OH), with added CaCl, (mo/L) 10. Average molar solubility of Ca(OH), with added CaCl, (mo/L) Account for the different molar solubilities in Part A.11 and Part B.10 (Report Sheet).arrow_forwardplz do correctly & show all work thnx!!! Solid lead(II) nitrate is slowly added to 150 mL of a 0.106 M potassium carbonate solution until the concentration of lead ion is 0.0358 M. The percent of carbonate ion remaining in solution is [ Submit Answer Retry Entire Group 6 more group attempts remaining Solid copper(II) nitrate is slowly added to 125 mL of a ammonium sulfide solution until the concentration of copper(II) ion is 0.0548 M. The maximum amount of sulfide remaining in solution is M. When 12.0 mL of a 6.35×104 M ammonium sulfide solution is combined with 25.0 mL of a 5.64×104M cobalt(II) nitrate solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal to[ When 15.0 mL of a 6.32x104 M nickel(II) iodide solution is combined with 25.0 mL of a 2.32×104M sodium hydroxide solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal toarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning