
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
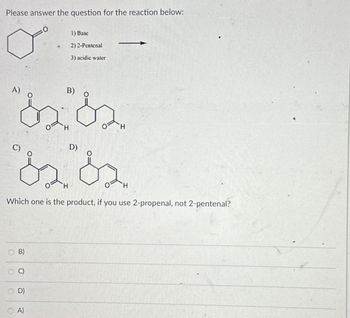
Transcribed Image Text:Please answer the question for the reaction below:
+
A)
B)
D)
1) Base
2) 2-Pentenal
3) acidic water
H
H
H
Which one is the product, if you use 2-propenal, not 2-pentenal?
O
Olo
O
B)
C)
D)
A)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Use the molecule show and draw the major products using the reagentsarrow_forwardWhat type of reaction occurs when a quantity of water is added to cyclohexene in the presence of an acid catalyst that results in the formation of an alcohol? a) Substitution reaction Ob) Addition reaction c) Esterification reaction d) Elimination reactionarrow_forwardGive the IUPAC name for each compound (except A)arrow_forward
- Complete the following reaction, and answer the questions below: OH (NaoH) a) What s the name of this reaction? b) OH in the above reaction is a base or a nucleophile ?arrow_forward16) The best classification for the following compound is: O 0 A) aldehyde B) ester C) ketone D) carboxylic acid 17) The name of the following compound: A) phenyl ethanoate B) phenyl propanoate C) propyl benzoate D) butyl benzoate 18) Complete the following reaction: CH3COOC₂H5 + NaOH- A) CH3COCH 3 B) CH3COCH₂CH3 C) CH3COONa D) CH3COOCH₂ من + HOC₂H5.arrow_forwardPredict the product of the following reaction. NaOH OH a) b) OH c) d) a darrow_forward
- (a) Rank the following molecules from most acidic to least acidic. Use the format 1>2 if you want to indicufe lis more acidic than 2. circle the molecules which can be >50% deprotonated in water nor 요.. 애 2 ii 3 A 4arrow_forwardHi, Need help with ii a & barrow_forwardGive the IUPAC name for each compound (a)-(e)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY