
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
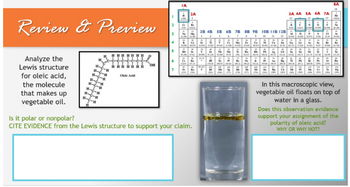
Transcribed Image Text:Review & Preview
Analyze the
Lewis structure
for oleic acid,
the molecule
that makes up
vegetable oil.
H.
H.
HH
-C-H
H
Olele Acid
OH
3
4
5
ΤΑ
2A
úte
Na Mg
22
3B 4B 5B 6B 7B 8B 98 10B 11B 128
8
9
A
22.
Is it polar or nonpolar?
CITE EVIDENCE from the Lewis structure to support your claim.
3A 4A SA 6A 7A
15
6
85404 47.62906422455 HA HEM 64
RON
1834 18621 1962 1922 14 167 2039 204.34 2022 2049
He
MI IN
1200
29
122250443206155
a
Zn Ga G
*AAAAAA**R7E
182.41 11482 11831 12:36 1270 1260 L
McLa
8A
16
In this macroscopic view,
vegetable oil floats on top of
water in a glass.
Does this observation evidence
support your assignment of the
polarity of oleic acid?
WHY OR WHY NOT?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2Onl x S Write electro X O uzdLQYhAS x G Predict the X Periodic Tab X b Answered: [ X 0 mySigTau genow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take [References] A compound, XF4, is 39.03% fluorine by mass. Identify the element X. What is the molecular structure of XF,?arrow_forwardHow many valence electrons should be present in their drawing?arrow_forwardplease explain in neat hand writingarrow_forward
- Which of the "arrowed" bonds in the molecule below is the longest ? Consult Periodic Table below. IA Η MILA Be 11 12 Na Ma 19 20 21 K Ca Sc Ti 3 E Rb Sr 40 Y Zr V Select one: OH O A. C-O O B. C-N O C. C-C B-H 41 42 43 44 45 40 47 40 Nb Mo Tc Ru Rh Pd Ag Cd 56 56 57 72 72 78 79 74 76 76 C Ba La Hf Ta W Re Os Ir Pt 104 106 100 101 108 109 110 Fr Ra Ac Ung Unp Unh Uns Uno Une Unn 87 O D. C-B IIIA IVA VA VIA VIIA He B C 13 14 A Si 24 20 27 28 29 30 31 32 33 34 35 36 Cr Mn Fe Co Ni Cu Zn Ga Ge As Cu Zn Ga Ge As Se Br Kr In VIILIA 10 N 0 F Ne 41 50 51 60 Au Hg Tl 15 16 17 18 P IS CI Ar 142 82 651 63 64 Te I Xe Sn Sb Te 82 83 64 84 65 Pb Bi Po At Rnarrow_forwardgeNSUINortheastern St Content OWLv2 LOnline teachina. Chomistry Questions& A. A Shert Danor: Alcohol/Foc O X Screenshot 2020-11-15 at 11.09... O O Q Q OC [References] A compound, XF4, is 39.03% fluorine by mass. Identify the element X. Scien Ch What is the molecular structure of XF4? trigonal pyramid Try Another Version 3 item attempts remaining Ch square pyramid Ch ahedron Ch trigonal bipyramid linear tetrahedron bent Ch plane triangle Ch Ch acy - Terms Ch 11:10arrow_forward21 Onl x S Write electrc X uzdLQYhASX x G Predict thet X t x Periodic Tab x b Answered: x 0mySigTau X M (no subject) > genow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take [References] Consider the following Lewis structure where E is an unknown element: :ö: 0-E-O :( What is a possible identity for element E? Predict the molecular structure (including bond angles) for this ion. Molecular shape is v with bond anglesarrow_forward
- Give detailed Solution with explanation needed with structure..don't give Handwritten answerarrow_forwardnd lea X G The element in period 5 that has X C Solved The following Lewis diagr X akeCovalentActivity.do?locator-assignment-take Lewis Structures Lewis Structures are used to describe the covalent bonding in molecules and ions. Draw a Lewis structure for NH3 and answer the following questions based on your drawing. 1. For the central nitrogen atom: The number of lone pairs The number of single bonds = The number of double bonds= 2. The central nitrogen atom A. Obeys the octet rule B. Has an incomplete octet. C. Has an expanded octet. Submit Answer [Review Topics] [References] Use the References to access important values if needed for this question. PRE + Retry Entire Group 9 more group attempts remaining Previous ENG Next> 11:55 AM 11/28/2022arrow_forwardA east.cengagenow.com e: CHM 103 003 General Chemistry I C OWLV2 | Online teaching and learning resource from Cengage Learning 6:43 [References] Use the References to access important values if needed for this question. 1 pt Which one of the following bonds is the most polar? (electronegativities: C = 2.5, O = 3.5, Cl= 3.0, N = 3.0, H = 2.1) 1 pt 1 pt O H-CI 1 pt O C-H N-CI 1 pt OC-CI 1 pt O0-0 1 pt Submit Answer Try Another Version 5 item attempts remaining 1 pt 1 pt 1 pt Cengage Learning Cengage Technical Supportarrow_forward
- Draw the Lewis structure for (NH4+)arrow_forwarddimensional structure of each molecule. Refer to the steps for drawing Lewis structures, the molecular geometry guide, the polarity flow chart and the attractive forces chart in the modules in the Unit 3 course documents to help you complete the chart. Molecular Total # of valence Formula electrons Ex: NCI3 CO₂ BF3 (exception to octet rule) H₂O N: 5 e Cl: 7e x 3 = 21 Total = 26 e Lewis Structure :d-N-a: 1 :cl: # of Electron Groups 4 28 Electron Geometry Bond Angle tetrahedral. 109.5 * # Bonds / # Lone Pairs 3/1 3 Molecular Geometry trigonal pyramidal Polar or Nonpolar polar ||| Attractive Force dipole- dipole O <arrow_forwardPlease don't provide handwritten solution ......arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning