
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
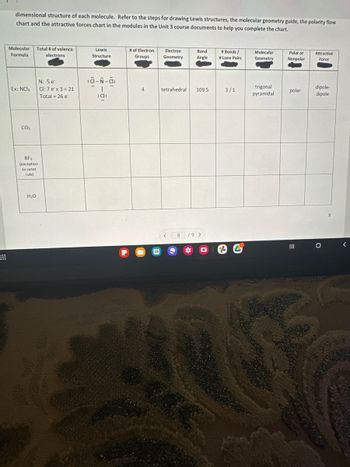
Transcribed Image Text:dimensional structure of each molecule. Refer to the steps for drawing Lewis structures, the molecular geometry guide, the polarity flow
chart and the attractive forces chart in the modules in the Unit 3 course documents to help you complete the chart.
Molecular Total # of valence
Formula
electrons
Ex: NCI3
CO₂
BF3
(exception
to octet
rule)
H₂O
N: 5 e
Cl: 7e x 3 = 21
Total = 26 e
Lewis
Structure
:d-N-a:
1
:cl:
# of Electron
Groups
4
28
Electron
Geometry
Bond
Angle
tetrahedral. 109.5
< 8 /9 >
*
# Bonds /
# Lone Pairs
3/1
3
Molecular
Geometry
trigonal
pyramidal
Polar or
Nonpolar
polar
|||
Attractive
Force
dipole-
dipole
O
<
Expert Solution
arrow_forward
Step 1: Introduction
The above problem can solved by using VSEPR theory or if you know the actual lewis dot structure of the compounds.
Electronic configuration of Hydrogen - 1s1, Boron - 1s22s1, Carbon -1s22s22p2, Oxygen -1s22s22p4, Fluorine -1s22s22p5.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following molecules have net dipole moments? For the molecules that are polar, indicate the polarity of each bond and the direction of the net dipole moment of the molecule. Consider bonds nonpolar if they have an electronegativity difference of 0.4 or lower. a Polarity of bonds in CH₂Cl₂ C-H: C- Cl; Submit Dipole with negative at Dipole with negative at H Nonpolararrow_forwardUsing VSEPR method find molecular structure of NO3 and O3arrow_forwardAnswer the questions in the table below about the shape of the periodate (10) anion. How many electron groups are around the central iodine atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central iodine atom? (You may need to use the scrollbar to see all the choices.) Continue 787 11 FILMADON FALCION POGING FEB 27 0 (choose one) linear bent T-shaped trigonal planar trigonal pyramidal square planar square pyramidal tetrahedral sawhorse trigonal bipyramidal octahedral 3 4 tv Ⓒ2023 M Sarrow_forward
- Determine the formula unit for the compound formed when each pair of ions interacts. Al³+ and CN: Ca²+ and SO: Lit and NO3: NH‡ and F¯:arrow_forwardll X b My Questions | bartleby Login | bartleby 101 Chem101 ぐ app.101edu.co Question 1 Draw a resonance structure that shifts a pi bond to a new position. Don't increase the number of atoms with formal charges. Include all hydrogen atoms in your structure. H. Atoms, Bonds an Drawing CHa :CHs: %3. 3D CH2 CH2 MacBook 0:0 3D 3Darrow_forwardMolecule Name Number of Lewis Dot Structure Polar or Shape Valence Nonpolar? Electrons BCl; (non- octet) SO3 SO- SO- CH4 CO (unusual) CHCI3 C,H6 C,H4 C,H2arrow_forward
- How much energy (in kJ) is required to separate (break) one mole of H-H bonds? Bond Length (pm) Energy (kJ/mol) H-H 74 436 H-C 106.8 413 H-N 101.5 391 H-O 97.5 467 O A) +436 kJ B) -436 kJ O C) +74 kJ D) -74 kJ E) 106.8 kJarrow_forwardE D To answer the questions, interpret the following Lewis structure for NH4+. + H-N-H 1 H H 1 $ 4 Submit Answer R 1. For the central nitrogen atom: The number of lone pairs = The number of single bonds = The number of double bonds= F 2. The central nitrogen atom 5 Retry Entire Group F6 T G Cengage Learning Cengage Technical Support A 6 8 r F6 MacBook Air H obeys the octet rule has an incomplete octet has an expanded octet & 7 F7 U J * 00 8 DII F8 Laht Values if needed for this q - ( 9 K F9 O ) 0 L F10 P Previous available 11 + Email Instructor Save = Nex 11 F12arrow_forwardPlease note that "geometry" refers to the molecular or ionic geometry. A. The Lewis diagram for SeOF2 is: :0-Se-F: | F: The electron-pair geometry around the Se atom in SeOF2 is There are lone pair(s) around the central atom, so the geometry of SeOF2 is B. The Lewis diagram for CH4 is: HICIH H-C-H The electron-pair geometry around the C atom in CH4 is There are lone pair(s) around the central atom, so the geometry of CH4 isarrow_forward
- Draw the lewis and 3D Structure for these. please be neat as possible.arrow_forwardO ELECTRONIC STRUCTURE AND CHEMICA... Deciding whether a Lewis... Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure [O=C-H]* :0: : CIC CI: [¤¤-6: 0/5 Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: 0 No, it has the right number of valence electrons but doesn't satisfy the octet rule. 000 The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: 0 Alia V No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* X Ar If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example,…arrow_forwardUse the electronegativity values provided to determine which, if any, of the statements below is/are TRUE H: 2.1 C: 2.5 O: 3.5 F: 4.0 Group of answer choices The C-F bond is polar The C-O bond is non-polar The H-F bond is ionic More than one of the above statements is TRUEarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY