
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Continuous Filtration Involving a Recycle Stream
The fresh feed F to the process is 10,000 lb/hr of a 40 wt % aqueous biomass in suspension. The fresh feed is combined with the recycled filtrate from the filter and fed to the evaporator, where water is removed to produce a 50 wt % Bio solution, which is fed to the filter. The filter produces a filter cake that is composed of 95 wt % dry biomass wet by a 5 wt % solution that in the lab proves to be composed of 55 wt % water with the rest dry biomass. The filtrate contains 45 wt % biomass.
a. Determine the flow rate of water removed by the evaporator and the recycle rate for this process.
b. Assume that the same production rate of filter cake occurs, but that the filtrate is not recycled. What would be the process feed rate of 40 wt % biomass then? Assume that the product solution from the evaporator still contains 50 wt % biomass in water.
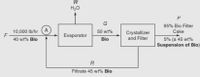
Transcribed Image Text:H2O
G
95% Bio Filter
A
10,000 lb/hr
50 wt%
Cake
Crystallizer
and Filter
Evaporator
Bio
5% (a 45 wt%
Suspension of Bio)
40 wt% Bio
R
Filtrate 45 wt% Bio
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- In the process of drying carrots using a parallel flow drying system, the material with a moisture content of 85% enters the drying system at a rate of 500 Kg / hour to produce dry carrots with a moisture content of 20%. If it is known that hot air used as the drying medium has a humidity ratio of 0.006 Kg of water per Kg of dry air and the flow of hot air enters the system at a rate of 200 Kg of air for each dry matter produced, calculate the ratio of humidity to air leaving the system assuming that the system is under steady state conditions.arrow_forwardOne way to make coal “cleaner” is to gasify the coal into “city gas”. In a proposed molten-iron coal gasification process pulverized coal of up to 3 mm size is blown into a molten iron bath and oxygen and steam are blown in from the bottom of the vessel. Materials such as lime for settling the slag, or steam for batch cooling and hydrogen generation can be injected at the same time. The sulfur in the coal reacts with lime to form calcium sulfide, which dissolves into the slag. The process operates at atmospheric pressure and 1400 to 1500°C. Under these conditions, coal volatiles escape immediately and are cracked. The carbon conversion rate is said to be above 98% and the gas is typically 65 to 70% CO, 25 to 35% hydrogen, and less than 2% carbon dioxide. Sulfur content of the gas is less than 20 ppm. Assume that the product gas is 68% Co, 30% H2, and 2% CO2. Calculate the enthalpy change that occurs on the cooling of 1000 m3 of the gas at 1475 °C and 1 atm to 25°C and 1 atm.arrow_forwardMagnesium metal, a gray solid, is heated in a crucible in the presence of oxygen. A white powder is collected from the crucible. This is an example of A) a chemical change B) a separation C) a mixture D) a physical changearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The