
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please see image
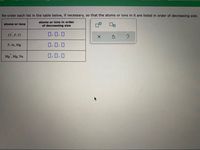
Transcribed Image Text:Re-order each list in the table below, if necessary, so that the atoms or ions in it are listed in order of decreasing size.
atoms or ions in order
of decreasing size
atoms or ions
CI, F, CI
0.0.0
P, Ar, Mg
0.0.0
Mg , Mg, Na
0.0.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chrome File Edit View History Bookmarks Profiles Tab Window Help 91% O Sun 10:58 O St. John's University - My Appl x A ALEKS - Iffat Khan - Knowledg x + i www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKZBYGfE9IMFjeRiThHre2U30XGpg1_cQJKNFZspY-BJWbSypPLYdk. Knowledge Check Question 8 Iffa The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant R. Suppose the osmotic pressure of a certain solution is measured to be 11. atm at an absolute temperature of 349. K. Write an equation that will let you calculate the molarity c of this solution. Your equation should contain only symbols. Be sure you define each symbol other than R. Your equation: Definitions of your symbols: D= 11. atm = 349. K IIarrow_forwardSorry if it's a lot but look at the picture please ?arrow_forwardChrome File Edit View History Bookmarks Profiles Tab Window Help N Watch Gilmore Girls | Netflix DashooardS A ALEKS - Reyna Garcia - Learn x A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZI6tTytly4Fcfu6zOtOf80MM9s8-pEjBx4anm2FVArDlj7Hnm8sjOBpH_25U... Apps Spotify Web Playe. M Common Ethical D. O CHEMICAL REACTIONS Writing a chemical equation from a description of the reaction 0/5 Solid iron(II) sulfide reacts with aqueous hydrochloric acid (HCI) to produce aqueous iron(II) chloride and hydrogen sulfide gas (H,S). Write a balanced chemical equation for this reaction. O-0 Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use 2 MacBook Airarrow_forward
- Which of the following is not a type of operating system? A) Real-time B) Network C) Distributed D) Sequentialarrow_forwardFill out the 2d plot shown in the picture along with all the labelsarrow_forwardrome File Edit View History Bookmarks Profiles Tab Window Help N Watch Gilmore x (5 unread) - dtE X AALEKS A ALEKS - David A ALEKS - Reyna A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZI6tTytly4Fcfu6zOtOf8oMM9sv O Spotify Web Playe.. M Common Ethical D.. O CHEMICAL REACTIONS Interconverting number of atoms and mass of compound Calculate the number of gold atoms in a 150.0 g sample of gold(III) chloride (Au,Cl). Be sure your answer has a unit symbol if necessary, and round it to 4 significant digits. Explanation Check APR 18 IIarrow_forward
- this question pleasearrow_forwardEdit View History Bookmarks Profiles Tab Window Help ome File d Dashboard ALEKS - Reyna Garcia - Lear Watch Gilmore Girls | Netflix A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZ16tTytly4Fcfu6zOtC Spotify Web Playe... M Common Ethical D... O CHEMICAL REACTIONS Writing a chemical equation from a description of the reaction Solid potassium and chlorine gas combine to produce solid potassium chloride. Write a balanced chemical equation for this reaction. Explanation Check FEB 2arrow_forwardMALEKS M Gmail ▬▬ 个 YouTube a X ALEKS - Rafia Riaz - Learn X Maps D Translate www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusWtY-MBckoyqj9qFtHEkTFh5e-b9D0Ng1h9QM42... Q O ACIDS AND BASES Predicting acid or base strength from the conjugate News species H₂O Explanation Bro Order these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. H₂0* HF Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. CH₂CH₂COOH b Answered: O ACIDS AND HBrO…arrow_forward
- me File Edit View History Bookmarks Profiles Tab Window Help Watch Gilmorex * Dementia Frien x 1 unread) - dte X Lobby Top Hat X 2 Dashboard xV Lives Well Livec x A ALEKS - David x Psychology Re i www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZ6tTytly4Fcfu6zOtOf8oMM9s8f0Bcp47VrzRTjh-0_CAFLD-fp-V... Spotify Web Playe.. M Common Ethical D.. O CHEMICAL REACTIONS Using a chemical equation to find moles of product from moles ... Dav Gaseous ammonia chemically reacts with oxygen (0,) gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of water produced by the reaction of 0.700 mol of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. Explanation Check © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardEdit View History Bookmarks Profiles Tab Window Help ALEKS = ALEKS - Tiffany Te-L-Peng - L X + www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-liGBdp5ulp5VqUnMDyPOVaX6q0CRPLZSQ5NeDIDYpEBUXdvV8IDfHNhBcWQZIM O ORGANIC OXIDATION AND REDUCTION Predicting the products of alkene ozonolysis X Predict the major products of this organic reaction. B Explanation Check c c C + T B 1.03 (CH3) S X MacBook Pro S Ć A Click and drag to start drawing a structure. O $ © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacarrow_forwardW AutoSave On chem - Compatibility Mode • Saved - O Search (Alt+Q) raghav grover RG File Home Insert Draw Design Layout References Mailings Review View Help P Comments A Share O Find Times New Roman v 12 - A A Aa v A Normal Body Text List Paragraph No Spacing E Replace Paste В I U ab x, x A - Dictate Editor Reuse A Select v Files Undo Clipboard s Font Paragraph Styles Editing Voice Editor Reuse Files Q6. Provide a step-wise synthesis for the following compound using benzene as a starting material. Br `NH2 K Accessibility: Unavailable D'Focus Page 4 of 13 523 words English (United States) 16 ENG 6:17 PM O Type here to search -16°C US 2022-03-02 近arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY