
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:File
Edit
View
History
Bookmarks
Profiles
Tab
Window
Help
? Q 2 0 Fr
ome
| Watch Gilmore Girl x
* Dementia Friend Ce X y Yahoo
Lobby Top Hat
ALEKS
A ALEKS - David Teac x Psychology Researx
A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH-IvdWKW_BBZZl6tTytly4Fcfu6zOtOf80MM9sjOAHGPMmOKRAJLU3Lxjedwm0V-. O ☆
R.
Pau
O Spotify Web Playe. M common Ethical D.
O CHEMICAL REACTIONS
1/3
David
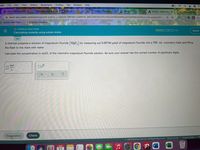
Calculating molarity using solute moles
A chemist prepares a solution of magnesium fluoride (MgF,) by measuring out 0.00744 µmol of magnesium fluoride into a 500. mL volumetric flask and filling
the flask to the mark with water.
Calculate the concentration in mol/L of the chemist's magnesium fluoride solution. Be sure your answer has the correct number of significant digits.
mol
x10
L
Explanation
Check
A
| Privacy Center
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use
MAR
11
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist prepares a solution of aluminum sulfate (Al, (So,)) by measuring out 191. g of aluminum sulfate into a 350. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's aluminum sulfate solution. Round your answer to 3 significant digits.arrow_forwardA chemist prepares a solution of potassium iodide (KI) by measuring out 260. g of potassium iodide into a 200. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's potassium iodide solution. Be sure your answer has the correct number of significant digits.arrow_forwardA chemist prepares a solution of mercury (I) chloride (Hg₂Cl₂) by measuring out 1.96 mg of mercury(I) chloride into a 500. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's mercury(I) chloride solution. Be sure your answer has the correct number of significant digits. mol/L x10 X Sarrow_forward
- A chemist prepares a solution of copper(II) sulfate (CuSO4) by measuring out 15. g of copper(II) sulfate into a 450. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's copper(II) sulfate solution. Be sure your answer has the correct number of significant digits.arrow_forwardA chemist prepares a solution of calcium sulfate (CaSO) by weighing out 0.293 g of calcium sulfate into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/L of the chemist's calcium sulfate solution. Be sure your answer has the correct number of significant digits. g x10 ?arrow_forwardSuppose 0.972 g of barium nitrate is dissolved in 50. mL of a 0.30 M aqueous solution of sodium chromate. Calculate the final molarity of nitrate anion in the solution. You can assume the volume of the solution doesn't change when the barium nitrate is dissolved in it. Be sure your answer has the correct number of significant digits. M x10 X Śarrow_forward
- please see attached imagearrow_forwardA chemist prepares a solution of barium chloride (BaCl,) by measuring out 17.1 g of barium chloride into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's barium chloride solution. Round your answer to 3 significant digits. I molLarrow_forwardA chemist prepares a solution of barium chloride (BaCl,) by measuring out 87. g of barium chloride into a 350. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's barium chloride solution. Round your answer to 2 significant digits. I molarrow_forward
- A chemist prepares a solution of nickel (II) chloride (NiCl2) by measuring out 160. umol of nickel (II) chloride into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's nickel (II) chloride solution. Round your answer to 3 significant digits.arrow_forwardPlease put the right amount of significant figuresarrow_forwardSuppose 2.92 g of copper(II) acetate is dissolved in 50. mL of a 0.60 M aqueous solution of sodium chromate. Calculate the final molarity of copper(II) cation in the solution. You can assume the volume of the solution doesn't change when the copper(II) acetate is dissolved in it. Be sure your answer has the correct number of significant digits. M x10 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY