
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
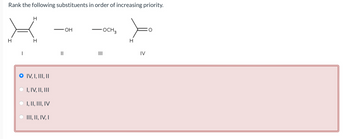
Transcribed Image Text:Rank the following substituents in order of increasing priority.
H
O IV, I, III, II
I, IV, II, III
I, II, III, IV
III, II, IV, I
OH
OCH 3
H
III
IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Rank the following groups in order of decreasing priority. −H, −CH3, −Cl, −CH2Clarrow_forwardRank each of the representations of NOCl from best to worst, placing the best structure on top and the worst at the bottom.arrow_forwardWhat is the configuration of following molecules: H ОО O COOH I -OH -OH I=RII=S I=S II=R I=S II=S I=R II=R II :0arrow_forward
- 2. Hemoglobin is a protein in red blood cells that contains iron and facilitates the transport of oxygen. The iron sits in the center of a heme ring in the form of Fe²+. The structure of a heme ring is here. H-C H-C H I H-C H H I C-N H-N-C C I C H C-N-H N-C C-H 1 C-H C-H H H a) The above structure is incomplete. Add electron pairs, non-zero formal charges, and/or additional bonds where needed to fulfill the octet rule to complete the structure. b) When Fe²+ is added to the structure in part a), the H atoms connected to the N are removed, see figure on the right. If you think of the heme ring as 4 N atoms, determine the molecular geometry of the new structure (you can draw it like [FeN4]2+). Imagine each N connects to Fe with a single bond. N- N Fe²+-N Narrow_forwardH₂C Li N(i-C3H7)2 CH3OCH₂OCH 3arrow_forwardSee Figure 9-6. The products of the E2 reaction of Compound P are A and B A and C A and D B and C B and D C and Darrow_forward
- Rank the following groups in order of decreasing priority. −NH2, −CH2NH2, −CH3, −CH2NHCH3arrow_forward7. Assign E or Z configuration to the following molecules: (A) (B) (C) (D) (E) I = Z; II = E I=E: II = Z I= E; II = E I=Z; II = Z I= E; II is neither E nor Z OH I II C1arrow_forwardDetermine the name of the following compound: CI (8S,9S,Z)-2,3,8,9-tetrachlorodec-2-ene (8S,9S,E)-2,3,8,9-tetrachlorodec-8-ene (8R,9R,Z)-2,3,8,9-tetrachlorodec-2-ene O (8S,9S,Z)-2,3,8,9-tetrachlorodec-8-ene ...arrow_forward
- Please be very confident in your answerarrow_forwardThe Newman projections of 1,1-dichloro-2-bromoethane are shown. H H H Br Br H H. Br ☀ ☀ ☀ A -H CI CI CI H Br A B D Select the most stable conformation(s). U A Barrow_forwardWhy are these two conformational isomers? I was under the impression that in molecules with only sigma bonds, the bonds can rotate freely, and so the placement of the chlorines wouldn't matter?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
