
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
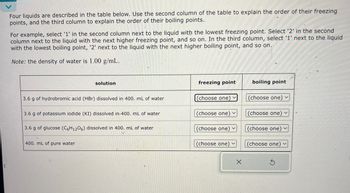
Transcribed Image Text:Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing
points, and the third column to explain the order of their boiling points.
For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second
column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid
with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on.
Note: the density of water is 1.00 g/mL.
solution
freezing point
boiling point
3.6 g of hydrobromic acid (HBr) dissolved in 400. mL of water
(choose one)
(choose one) v
3.6 g of potassium iodide (KI) dissolved in 400. mL of water
3.6 g of glucose (C6H12O6) dissolved in 400. mL of water
(choose one) v
(choose one)
(choose one)
(choose one) v
400. mL of pure water
(choose one)v
(choose one) v
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- PROVISIONAL LEWIS STRUCTURES T01/S01 For each provisional structure, identify if it is the correct Lewis structure (C) or if it needs to be modified according to procedure MA or MB to obtain the correct Lewis structure. MA. Form multiple bonds to the central atom using lone pair on the terminal atom(s) until the central atom has an octet (or septet). MB. Form multiple bonds to the central atom using lone pair on the terminal atom(s) until the central atom has a formal charge of zero (0) or until two double bonds are formed. A provisional structure has the valence electrons distributed in order as (1) a bond between each pair of atoms, (2) an octet for each ligand (terminal) atom or ligand group (CH3, NH2 or OH) and (3) any remaining electrons on the central atom.arrow_forward(1) Br2 CC,arrow_forwardLe Chateliers principle. 7 / + 3 Beg) = 2 A#= +60,000 Inc [B] Dec [D] Inc [C] €² 2 C + D (2) до Inc. I Дес Рarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY