
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
All I need is the full reaction and the net ionic equation
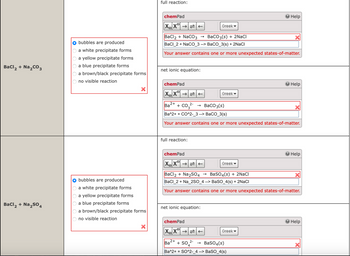
Transcribed Image Text:bubbles are produced
a white precipitate forms
a yellow precipitate forms
a blue precipitate forms
BaCl2 + Na2CO3
a brown/black precipitate forms
no visible reaction
BaCl2 + Na2SO4
OOOOO
full reaction:
chemPad
Greek▾
BaCl2 + NaCO3
→> BaCO3(s) + 2NaCl
Help
BaCl_2+ NaCO 3 --> BaCO_3(s) + 2NaCl
Your answer contains one or more unexpected states-of-matter.
net ionic equation:
chemPad
Help
Greek▾
2+
Ba
+ CO2-
->
BaCO3(s)
Ba^2++ CO^2-3 --> BaCO_3(s)
Your answer contains one or more unexpected states-of-matter.
full reaction:
chemPad
XX
Greek▼
Help
bubbles are produced
a white precipitate forms
a yellow precipitate forms
a blue precipitate forms
a brown/black precipitate forms
no visible reaction
BaCl2 + Na2SO4 → BaSO 4 (s) + 2NaCl
BaCl 2 + Na_2SO_4 --> BaSO_4(s) + 2NaCl
Your answer contains one or more unexpected states-of-matter.
net ionic equation:
chemPad
Help
XX
Greek▾
2+
Ba
2-
+504
→> BaSO4(s)
Ba^2++ SO^2-_4 --> BaSO_4(s)

Transcribed Image Text:bubbles are produced
a white precipitate forms
a yellow precipitate forms
BaCl2 + H2SO4
a blue precipitate forms
a brown/black precipitate forms
no visible reaction
BaCl2 + CuSO4
full reaction:
chemPad
XX"
Greek▾
BaCl2 + H2SO4
→>
BaSO4(s) + 2HCI
Help
BaCl_2+ H_2SO_4 --> BaSO_4(s) + 2HCI
Your answer contains one or more unexpected states-of-matter.
net ionic equation:
chemPad
Help
Greek
XX
2+
Ba
+50
2-
-> BaSO4(s)
Ba^2++ SO^2-_4 --> BaSO_4(s)
Your answer contains one or more unexpected states-of-matter.
full reaction:
chemPad
XX
Greek▾
Help
bubbles are produced
a white precipitate forms
a yellow precipitate forms
|BaCl2 + CuSO4 → BaSO4(s) + CuCl2
BaCl_2+ CuSO_4 --> BaSO_4(s) + CuCl_2
Your answer contains one or more unexpected states-of-matter.
a blue precipitate forms
a brown/black precipitate forms
no visible reaction
net ionic equation:
chemPad
Help
Greek▾
XX
2+
Ba'
+ SO →>
2-
BaSO4(s)
4-
Ba^2+ + SO^2- 4- --> BaSO 4(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- How many liters of 0.0300 M NaOH solution can be prepared from 2.00 g of NaOH?arrow_forwardBy titration it is found that 114.3mL of 0.562M NaOH is needed to titration 48.0mL of Hcl. what is the concentration of the HCl in molarity?arrow_forwardA student weighs out a 3.48 g sample of manganese(II) chloride, transfers it to a 125 mL volumetric flask, adds enough water to dissolve it and then adds water to the 125 mL tic mark. What is the molarity of MnCl, in the resulting solution?arrow_forward
- Predict the precipitation reaction(write molecular, ionic, and net ionic equations): CaCl, + AGNO;arrow_forwardConsider two aqueous solutions: one of ammonium carbonate and one of barium nitrate. List the ions present in each. Is a precipitate formed when the two solutions are mixed? If so, write the molecular, ionic and net ionic equation. Identify the spectator ionsarrow_forwardSonomic acid is a monoprotic acid developed in the chemistry department of Sacramento state University. The storeroom staff successfully obtained a sample of sonomic acid and made a solution. They made the solution by adding 302.4 g of sonomic acid and diluting it to a volume of 10.00 L with water. Unfortunately, the stockroom attendant, Steven , does not know the molar mass of sonomic acid so he could not calculate the molarity of the solution he prepared. It would be great if you could help him.arrow_forward
- When 4.96 g of KHP reacts with 1.7 M NaOH, the mL of NaOH required isarrow_forwardI'm stuck on a homework question. Any help would be greatarrow_forward3) When excess sliver nitrate was added to 25.00 ml of barium chloride, a precipitate of sliver chloride formed at the bottom of the beaker. If the mass of the precipitate was 4.30g, calculate the initial concentration of barium chloride.arrow_forward
- If 10. g of AgNO3 is available, what volume of 0.35 M AgNO3solution can be prepared?arrow_forwardsolid calcium sulfite + acetic acid net ionicarrow_forwardConsider the following reaction:2HCl+CaCO3→CaCl2+H2O+CO22HCl+CaCO3→CaCl2+H2O+CO2How many mols of calcium chloride can be produced if you begin with 14.47 mL of 0.62 M HCl and 7.24 grams of calcium carbonate?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY