
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
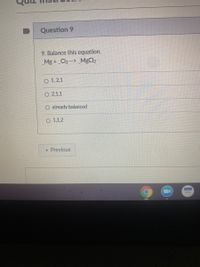
Transcribed Image Text:Question 9
9. Balance this equation.
_Mg + _Cl2 --> _MgCl2
O 1, 2,1
O 2,1,1
O already balanced
O 1,1,2
* Previous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- mol M A solution is made from 0.315 mol of Na Cr207. If the volume of the solution is 25mL, what is the molarity of the solution? O 0.0126 M O 12.6 M O 0.0794 M O 79.4 M hp # & 2 3 4. e f C V lo d'arrow_forwardGreat work! 3. Pick a "for every" statement about copper and silver in this balanced reaction. 29 47 Cu Ag Copper 63.55 Silver 107.87 Hint: The correct balanced equation is Cu + 2A9NO, → 2Ag + Cu(NO,)2 For every 1 mole of copper, the reaction makes 2 moles of silver. For every 1 mole of copper, the reaction uses up 2 moles of silver. For every 1 mole of copper, the reaction makes 1 mole of silver. Submit Answer 3/3 submissions remaining Score:arrow_forwardQ 34arrow_forward
- balance this chemical reaction _____- C2H4O + _____ O → ______C2H4O2arrow_forwardMasses : Ca = 40.08 g S= 32.07 g O= 16.00 g A 35.0 mL sample of CaSO4 was evaporated to dryness, leaving 0.967 g of residue. What was the molarity of the original solution? step by step pleasearrow_forwardBalance the following unbalanced chemical equation showing the combustion of methane. _______ CH4 + ________ O2 --> _________ CO2 + _________ H2Oarrow_forward
- * 0 il * CE Previous Page 4 of 9 Mass = 12 S Next → g S CHAPTER 4-STOICHIOMETRY: QUANTITATIVE INFOR S What mass of oxalic acid, H₂C2O4? H₂C2O4, is required to prepare 100. mL of a solution that has a concentration of 0.40 M alı 4 C Document3.docx C RESEA Carrow_forward1. How do you distinguish reactants and products of a chemical equation? 2. What should you do if the reactants and products are balanced/equal to one another? 3. Na + Cl2 ------> NaCl Solve. 4. Ag2S ------> Ag + S8 Solve.arrow_forwardWhat type of reaction does the illustration below represent? a decomposition single replacement double replacement d. synthesis Question 2 The complete balanced equation for the reaction between sodium hydroxide (NAOH) and magnesium sulfate (MgS04) is: a 2 N2OH + MgSO4 -> Mg(OH)2 + Na2SO4 NaOH + MgSO4 -> Mg(OH)2 + NaSO4 NAOH + MgS04 -> Na + Mg + H2SO4 d NaOH +MgS04 -> NaMgSO4 + H2Oarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY