
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
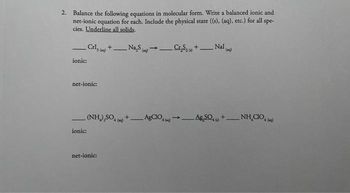
Transcribed Image Text:2. Balance the following equations in molecular form. Write a balanced ionic and
net-ionic equation for each. Include the physical state ((s), (aq), etc.) for all spe-
cies. Underline all solids.
—
_Cris (2) +Na₂S (g) →.
ionic:
net-ionic:
ionic:
_C₂S+_ Nal()
net-ionic:
-
(NH),SO, +__AgCIO, → AgSO, +__NH
CO,
->
(mg)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Macmillar For the chemical reaction HCN(aq) + KOH(aq) → H,O(l) + KCN(aq) write the net ionic equation, including the phases. net ionic equation:arrow_forwardSodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO, neutralizes excess HCI through this reaction: HCl(aq) + NaHCO3(aq) NaCl(aq) + H₂O(1) + CO₂(g) The CO₂ gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a man suffering from indigestion can be considered to be 200 ml of a 0.050 M HCI solution. What mass of NaHCO, would he need to ingest to neutralize this much HCI? Be sure your answer has the correct number of significant digits. Xarrow_forward6 a b U d [Tutorial: Titration stoichiometry] This question will walk you through the steps of calculating the volume of titrant required to reach the endpoint based on the volume and concentration of analyte. Consider the standardization of NaOH by titration with C,H,O, according to the following chemical reaction: 3 NaOH(aq) + C,H,O,(aq) = Na,C,H,O,(aq) + 3 H₂O(l) Step 1: Evaluate the question. How many moles of aqueous sodium hydroxide (NaOH) will react to completely neutralize one mole of aqueous citric acid (C,H,O,)? Which equation will be most helpful for solving this problem? Calculate the volume in mL of a 0.200 M NaOH solution needed to neutralize 345 mL of 0.033 MC,H,O, standard solution. Based on the fact that NaOH is a strong base and C,H,O, is a weak acid, how would you describe the pH of the solution at the endpoint, where moles of base = 3 times the moles of acid?arrow_forward
- Write the net ionic equation for the following: CaCla + NazCO; * Co.*- (aq) + Ca²* (aq) CACO: (s) Na (aq) + Cl- (aq)NaCl(s) Ca2+ (aq) + Na* (aq) – CaNa: (s) no precipitate forms Which of the following ionic reaction represents a complete ionic equation? I. Al,0, (s) + 6H* (aq) + 6C1O, (aq) → 2 Al3*(aq) + 6C10, (aq) +3 H, 0 (I) II. PbCr0, (s) → Pb2+(aq) + Cr0; (aq) III. Mg(OH)2 (s) + H2SO, (aq) → MgSO, (aq)+ 2 H20 (1) IV. H2CO, (aq) + 20H (aq)→ clo;- (aq) + 2 H,0 (I) II IVarrow_forwardSodium hydrogen carbonate (NaHCO,), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI) , which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO, neutralizes excess HCl through this reaction: HCl(aq) + NaHCO;(aq) → NaCl(aq) + H,O(1) + CO,(g) The CO, gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a man suffering from indigestion can be considered to be 100. mL of a 0.048 M HCI solution. What mass of NaHCO, would he need to ingest to neutralize this much HCl ? Be sure your answer has the correct number of significant digits. x10arrow_forward17. Predict whether or not a precipitation reaction will occur if the pairs of solutions that are given below are mixed. If you think a reaction will occur, write a balanced equation for the precipitation reaction. Na,S0,(aq) + K2CO3(aq)→ К,со, (аq) + Са(NO;3)2(aq) > СаCl2(aq) + NaNO3(аq) — СаCl2(aq) + 2AgNO,(aq) —arrow_forward
- 4. Calculate the calculate the mass of precipitate formed from 5.0 g of strontium chloride. Li3PO4(aq) → Sr3(PO4)2(S) + LiCl(aq) _SrCl₂(aq) + 5. What mass of aluminum hydroxide in needed to neutralize 5.00 g of hydrochloric acid? Al(OH)3(s) + _HCl(aq) → ___AlCl3(aq) + _H₂O(1) 195arrow_forwardWhich of the below represents the complete ionic equation for the reaction (if any) that occurs when aqueous solutions of iron(II) nitrate and potassium sulfide are mixed? Group of answer choices Fe2+(aq) + S2-(aq) → FeS(s) K+(aq) + NO3-(aq) → KNO3(s) 2 K+(aq) + S2-(aq) + Fe2+(aq) + 2 NO3-(aq) → Fe2+(aq) + S2-(aq) + 2 KNO3(s) No reaction occurs. 2 K+(aq) + S2-(aq) + Fe2+(aq) + 2 NO3-(aq) → FeS(s) + 2 K+(aq) + 2 NO3-(aq)arrow_forwardSodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl(aq) + NaHCO3(aq) → NaCl(aq) + H2O(l) + CO2(g) ->> The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a man suffering from indigestion can be considered to be 100. mL of a 0.089 M HCl solution. What mass of NaHCO3 would he need to ingest to neutralize this much HCI? Round your answer to 2 significant digits. x10 xarrow_forward
- During a lab experiment performed at STP conditions, you prepare HCl by reacting 100. ml of Cl2 gas with an excess of H2 gas. How many ml of a solution of Ba(OH)2 0.230M do you need to neutralize all the HCl produced? Please show solution in one string using a dimensional analysis step-by-step.arrow_forward1. (a) Write balanced molecular equations for the following potential precipitation reactions. Indicate the states of reactants and products [(aq) or (s)]. (b) In those cases where a precipitate forms, write the net ionic equation. If there is no reaction, state "No reaction." (i) Na₂S (aq) + Pb(NO3)2 (aq) → (iii) MgCl₂ (aq) + Pb(NO3)2 (aq) → (ii) FeSO4 (aq) + Na3PO4 (aq) → (iv) K₂SO4 (aq) + Ni(NO3)2 (aq) →arrow_forwardCould you please balance each equation and tell me what type of equation it isarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY