
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
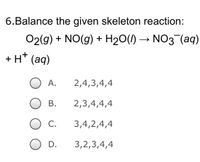
Transcribed Image Text:6.Balance the given skeleton reaction:
O2(g) + NO(g) + H₂O(l) → NO3¯(aq)
+ H+ (aq)
2,4,3,4,4
2,3,4,4,4
3,4,2,4,4
3,2,3,4,4
A.
B.
C.
D.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- AaBbCcDc AaBbCcDc AAB6CC AaBbCcD AaB 三 . 1 Normal 1 No Spac.. Heading 1 Heading 2 Title Paragraph Styles 2) Four metals (A, B, C, and D) were studied for activity, and the following information was collected on the reactions between the metals and their salts: I) 2 A (s) + C(NO3)2 (ag) → No Reaction II) C (s) + 2 BCI (ag) → CC12 (ag) + 2 B (s) III) D (s) + B2S04 (ag) → DSO4 (ag) + 2 B (s) IV) 2 B (s) + 2 HCI (ag) → BC12 (ag) + H2 (g) V) D (s) + 2 AÑO3 (ag) → No Reaction a) Based on the above results, indicate the more reactive metal for each reaction: Most reactive> least reactive b) Would you expect A to react with sulfuric acid? Explain. 3)arrow_forward1. A 125 mL sample of orange juice was titrated using a redox reaction to the equivalence point with the addition of 7.6 mL of a 0.0025M iodine (I) solution. What is the concentration of vitamin C (CH,O) in this sample? (Note that 1,000 mM = 1 M) a. 30 mM b. 15 mM c. 19 mM CHO (aq) + (aq) → CHO (aq) + 21 (aq) + 2H (aq) ->>> d. 0.30 mM e. 0.15 mMarrow_forwardFor the following redox reactions, please balance them in basic solution and indicate the impact of pH on the reaction (e.g., lower pH favors, disfavors, has no effect on the reaction).arrow_forward
- 8 H+(aq) + Cr2O72-(aq) + 3 H2S (aq)----> 2 Cr+3 (aq) + 7 H2O (l) + 3 S (s)a. Assign oxidation numbers to each element in each substance.b. What is the oxidizing AGENT? What is the reducing agent?arrow_forwardBalance the following using the half-reaction method. Identify oxidation, reduction, reducing agent, oxidizing agent. i. Be(NO3)2 (aq) + Ce(s)--> Ce(NO3)3(aq) + Be(s) ii. A container contains H2SO3, H2SO4, Pb2+, Pb4+ and NO3- in distilled water. Create a balanced chemical reaction (Net Ionic Equation, including states of matter) for the spontaneous reaction in the containerarrow_forwardBalance the following Redox Reaction. Always include the state of each species: (s), (l), (g), or (aq). O2(g) + Sb(s) → H2O2(l) + SbO2-(aq) (basic) Format: A. Write the Balanced Oxidation Half Reaction B. Write the Balanced Reduction Half Reaction C. What is the Reducing agent? Oxidizing agent? D. Write the overall balanced equation.arrow_forward
- 1- Balance the following redox reactions using the oxidation number method a. NaClO + H2S = NaCl + H2SO4 b. Sn + HNO3 + H2O = H2SnO3 + NO 2- Balance the following redox reactions using the half-reaction method. a. Mg + Al+3 = Mg+2 + Al b. H2O2 + Cr2O72- = O2 + Cr+3 (in acidic solution) 2.Rank the following entities in order of increasing strength as oxidizing agents. (1=weakest oxidizing agent and 6= strongest oxidizing agent) Cd+2(aq) IO3-1(aq) K+1(aq) H2O(l) AuCl4-1(aq) I2(s) 3. Indicate which of the following pairs of substances, when combined, will result in a spontaneous chemical reaction. Write spontaneous or non-spontaneous. a. K+1(aq) and Na(s) b. Cr+2(aq) and K(s) c. Pb+2(aq) and Fe(s) d. Ag(s) and Sn+2(aq)arrow_forwardComplete and balance the reaction between calcium carbonate and hydrochloric acid. Then give the correct product side of the reaction. Choose one: a. → CaCl2 (aq) + H2CO2 (aq) b. none of these c. → CaCl2 (aq) + H2O (l) + CO2 (g) d. → 2 CaCl (aq) + H2CO2 (aq)arrow_forwardFor the following redox reactions, please balance them in basic solution and indicate the impactof pH on the reaction (e.g., lower pH favors, disfavors, has no effect on the reaction).arrow_forward
- Identify the reducing and oxidizing agents and determine the species that is oxidized and the species that is reduced. Mg Fe2+ Mg 2+ a. Fe b. Cu2+ Sn Sn2+ Cu с. H2 CH2=CH2 CH3CH3 Reducing agent Oxidizing agent Species being reduced Species being oxidizedarrow_forward100arrow_forwardA. +2 13. In the reaction, 2H₂ (g) + O₂ (g) → 2H₂O (g), which of the following is the oxidizing agent? C. H₂O B. 0₂ A. H₂ D. None of the choices 14. In the reaction, 2H₂ (g) + O₂(g) → 2H₂O (g), which of the following is the reducing agent? C. H₂O A. H₂ B. 02 D. None of the choices 15. What are the original and final oxidation numbers for iron in the smelting of iron from iron oxide? Fe2O3(s) + 3CO(g) → 2Fe(s) + 3C02(g) C.0 +2 A. +2 0 B. +3-0 D. No change 16. A reducing agent reduces other substances and loses electrons; therefore, its oxidation state will C. stay the same B. increase A. decrease ? D. none of the choices 17. A monoatomic ion has an oxidation state equal to its charge. What is the oxidation state of Ca²+? A. +2 B. +1 C.0 D.-2 18. In the reaction, Fe2O3 (1) + CO (g) → Fe (I) + CO2 (g), how many electrons does Fe gain? A. 1 B. 2 C. 3 D. 4 19. In the reaction, Fe2O3 (1) + CO (g) → Fe (1) + CO2 (g), how many electrons does Fe gain? A. 1 B. 2 C. 3 D. 4 20. Which of the…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY