
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:QUESTION 4
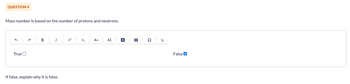
Mass number is based on the number of protons and neutrons.
G
True
B
1
If false, explain why it is false.
x²
X₂
Aa A$
A
H
Ω
14
False✔
Expert Solution
arrow_forward
Step_1
Number of protons = p
Number of neutrons = n
Mass number = A
A = p+n
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- QUESTION 9 _was the first to calculate how much more energy an atom release in radioactive decay that it does in any chemical reaction. J.J.Thompson W.Roentgen Marie Curie O F. Soddyarrow_forwardV2arrow_forwardQUESTION 1 Aston's measurements with mass spectrograph showed that the mass of the carbon-12 nucleus _the mass of 12 hydrogen nuclei. was slightly less than, exactly equal to slightly more than O none of thisarrow_forward
- Question 26 3.3pts Doctors nationally believe that 78% of a certain type of operation are successful. In a particular hospital, 52 of these operations were observed and 42 of them were successful. At a = 0.05 is this hospital's success rate different from the national average? O Yes, because the test value 0.28 is in the critical region. O No, because the test value 0.28 is in the noncritical region. O No, because the test value 0.48 is in the noncritical region. O Yes, because the test value 0.48 is in the noncritical region.arrow_forwardQuestion 18 of 20 View Policies Current Attempt in Progress When any radioactive dating method is used, experimental error in the measurement of the sample's activity leads to error in the estimated age. In an application of the radiocarbon dating technique to certain fossils, an activity of 0.110 Bq per gram of carbon is measured to within an accuracy of 9.00 percent. Find (a) the age of the fossils and (b) the maximum error (both in years) in the value obtained. Assume that there is no error in the 5730-year half-life of C¹4 nor in the value of 0.23 Bq per gram of carbon in a living organism (a) Number (b) Number Save for Later Units Units Attempts: 0 of 1 used Submit Answerarrow_forwardCalculate unknown reactions and draw the FBDarrow_forward
- Using values from this table, how many DNA molecules could be broken by the energy carried by 1 kg of hydrogen via fusion to helium? molecules Additional Materials Reading +.arrow_forward2 / 2 221% The following system Radioactive Decay Series of differential equations is encountered in the study of the decay of a special type of radioactive series of elements: dx = - Ajx dt %3D dy = Ax - 2y, dt where A and A, are constants. Discuss how to solve this system subject to x(0) = x0, y(0) = yo- Carry out your ideas. %3Darrow_forwardYou are experimenting with a radioactive sample of polonium. At the end of 14.0 minutes, exactly 1/16 of the polonium remains. Part A Approximately how much more time you will need to wait until less than 0.1% of the original polonium sample remains? 21 minutes 1 hour and 10 mins 3 hours and 30 mins 35 minutes Submit Request Answerarrow_forward
- A radioactive sample has a half-life of 1.0 min. That is, each nucleus has a 50% of undergoing a decay sometime between t = 0 and t = 1 min. One particular nucleus has not decayed at t = 15 min. Part A What is the probability that this nucleus will decay sometime between t = 15 min and t = 16 min? Express your answer using two significant figures. ΤΟ ΑΣΦ ? %arrow_forwardThe neutron was found in the laboratory in what year? a. 1788 b. 1849 c. 1888 d. 1932 e. 1970arrow_forwardQuestion A8 Sketch a diagram indicating the s-process path from 131 Xe to 140 Ce. Specify the type of each process (i.e. neutron capture, electron capture, beta decays) in the path.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON