Question
how would you do part b of this question? this is a non graded practice worksheet
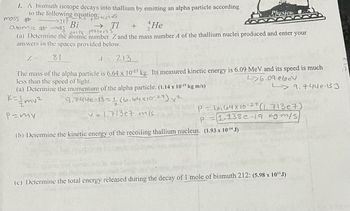
Transcribed Image Text:1. A bismuth isotope decays into thallium by emitting an alpha particle according
to the following equation:
→217
mass #
All particles
atomic #83
Bi
He
only
protons
(a) Determine the atomic number Z and the mass number A of the thallium nuclei produced and enter your
answers in the spaces provided below.
Z == 81
1 = 213
The mass of the alpha particle is 6.64 x 10-27 kg. Its measured kinetic energy is 6.09 MeV and its speed is much
less than the speed of light.
26.09 ebev
(a) Determine the momentum of the alpha particle. (1.14 x 10-¹9 kg m/s)
K=//mv²
9.744e-13=116.64x10-27) ²
P=my
→ TI +
0.00P dige
v=1.713e7 m/s
Plyysics
(b) Determine the kinetic energy of the recoiling thallium nucleus. (1.93 x 10-14 J)
P - 6,64 x 10-27 (1. 713e7)
P
1.138e-19 kgm/s)
9.744e-13 J
pretopte
(c) Determine the total energy released during the decay of 1 mole of bismuth 212: (5.98 x 10¹¹J)
ora
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Similar questions
- Hello, can you also please include the formula that you used to solve this problem? Thank you!arrow_forwardPlease answer question and just send me the paper solutions asap dont type the answer question and just ex2 answer all the parts please faster and use the equation from picture please asaparrow_forwardhow would you answer this questions? it is a non graded practice assignmentarrow_forward
- Physics sample problems about vectors. Thought I knew how to do them but keep getting wrong answers.arrow_forwardCan you please answer number 1 and all of the sub questions and show all of the steps to the solutionarrow_forwardhow would you do this? this is a non graded practice worksheetarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios