
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
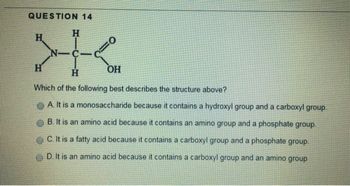
Transcribed Image Text:QUESTION 14
H
N-
H
=0
H
OH
H
Which of the following best describes the structure above?
A. It is a monosaccharide because it contains a hydroxyl group and a carboxyl group.
B. It is an amino acid because it contains an amino group and a phosphate group.
C. It is a fatty acid because it contains a carboxyl group and a phosphate group.
D. It is an amino acid because it contains a carboxyl group and an amino group
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- QUESTION 41 Which of the following best summarizes the relationship between condensation reactions and hydrolysis reactions? O a. Condensation reactions require the addition of water while hydrolysis reactions release water. O b. Condensation reactions assemble monomers into polymers and hydrolysis reactions break down polymers. O c. Hydrolysis reactions create monomers and condensation reactions break down polymers. O d. Condensation reactions create monomers and hydrolysis reactions assemble monomers into polymers. e. a and c Click Save and Submit to save and submit. Click Save All Answers to save all answers. 2 F2 # 3 30 F3 $ 4 F4 % 5 MacBook Proarrow_forwardQUESTION 45 When can we say that atom has no vacancy, or the atom is full? O a. An atom's outer shell is filled with protons O b. An atom's outer shell is filled with electrons O C. An atom's outer shell is filled with neutrons O d. An atom's inner shell is filled with electrons O e. An atom's inner shell is filled with protons QUESTION 46 Lipids O a. include fats consisting of three fatty-acid molecules and one glycerol molecule O b. are composed of monosaccharides OC. are only found in animals O d.include cartilage and chitin O e. yield less energy per gram than do carbohydrates QUESTION 47 Fats provide more energy than carbohydrates because they O a. are liquid, thus requiring less energy for reactions O b.are partially oxidized initially, thus requiring less ATP for oxidation O C. require more oxidation reactions to break down, thus releasing more electrons O d. are hydrophilic, thus entering cells easily O e. can be stored inside the cell for long periods of timearrow_forwardQuestion 22arrow_forward
- Can you please solve the following question please.arrow_forwardQuestion 2. Which of the following is a molecule formed by a dehydration synthesis reaction? A. Fatty acid B. polypeptide C. Glycerol D. ATP E. Glycinearrow_forwardQUESTION 9 What type of bond holds protein monomers (building blocks) together? A. ionic O B. covalent O C. peptide. O D. hydrogenarrow_forward
- Can you please solve the following question please.arrow_forwardQuestion 21 Two macromolecules, such as proteins, can adhere tightly and specifically to each other. How can weak intermolecular forces lead to such strong adherence? O the weak forces are readily converted to covalent bonds, thus leading to strong adherence between molecules O if the weak forces are correctly aligned they can become as strong as covalent bonds O adhesion is strong because the weak forces can be involved in condensation reactions O the weak forces can become very strong once non-polar groups are excluded from the inside of the molecules O adherence can be quite strong by having many weak forces involved in molecular adhesion • Previousarrow_forwardQuestion 2. An enzyme was found to convert Acetyl-coA to an acetyl-cysteine intermediate of the enzyme. The structure of cOA is: H H НО СН; HS — СH2— СH2 — N—C—CH,— CH2—N- С—С—С—CH;—0—Р- 0–CH2 Adenine н CH H H H Phosphopantetheine group of coenzyme A 2-O3PO ОН Draw a plausible chemical mechanism. Where is the acetyl-group in acetyl-coA? What is the reactive group of the protein? The reaction is enhanced at higher pH. How is this consistent with your mechanism? Is covalent catalysis involved?arrow_forward
- QUESTION 7 What is the relationship between the two structures shown? *-** Br H. Br H. and H. CI H. O same compound O enantiomers O diastereomers O none of thesearrow_forwardQUESTION 10 Which of the following types of bonds are present in the primary structures of proteins? O Hydrogen bonds and covalent bonds O Covalent bond O Covalent bonds, hydrogen bonds and dislufide linkages O lonic interactionsarrow_forwardQuestion #12arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education