
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
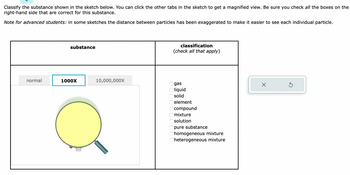
Transcribed Image Text:Classify the substance shown in the sketch below. You can click the other tabs in the sketch to get a magnified view. Be sure you check all the boxes on the
right-hand side that are correct for this substance.
Note for advanced students: in some sketches the distance between particles has been exaggerated to make it easier to see each individual particle.
normal
substance
1000X
10,000,000X
000
classification
(check all that apply)
gas
liquid
solid
element
compound
mixture
solution
pure substance
homogeneous mixture
heterogeneous mixture
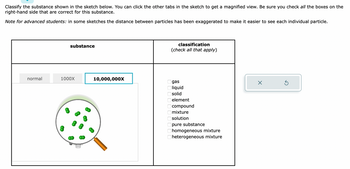
Transcribed Image Text:Classify the substance shown in the sketch below. You can click the other tabs in the sketch to get a magnified view. Be sure you check all the boxes on the
right-hand side that are correct for this substance.
Note for advanced students: in some sketches the distance between particles has been exaggerated to make it easier to see each individual particle.
normal
substance
1000X
10,000,000X
classification
(check all that apply)
8 8 8 8 8 8 8 8 8 8
gas
liquid
solid
element
compound
mixture
solution
pure substance
homogeneous mixture
heterogeneous mixture
X
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
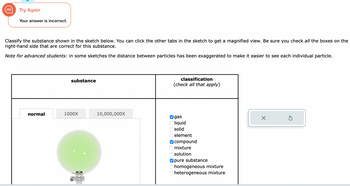
Transcribed Image Text:Classify the substance shown in the sketch below. You can click the other tabs in the sketch to get a magnified view. Be sure you check **all** the boxes on the right-hand side that are correct for this substance.
*Note for advanced students:* in some sketches the distance between particles has been exaggerated to make it easier to see each individual particle.
| **substance** | **classification** (check all that apply) |
|--------------------------------------|--------------------------------------------|
| ! Normal view diagram with particles displayed | ☑ gas <br> ⬜ liquid <br> ⬜ solid <br> ⬜ element <br> ☑ compound <br> ⬜ mixture <br> ⬜ solution <br> ☑ pure substance <br> ⬜ homogeneous mixture <br> ⬜ heterogeneous mixture |
Diagram Explanation:
- The diagram visually represents gas particles. The configuration illustrates the particles dispersed evenly with space between them.
- Three magnification options are available: Normal, 1000X, and 10,000,000X, although only the normal view is shown here.
Checklist:
- The correct classifications in this case are "gas," "compound," and "pure substance."
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question

Transcribed Image Text:Classify the substance shown in the sketch below. You can click the other tabs in the sketch to get a magnified view. Be sure you check **all** the boxes on the right-hand side that are correct for this substance.
*Note for advanced students:* in some sketches the distance between particles has been exaggerated to make it easier to see each individual particle.
| **substance** | **classification** (check all that apply) |
|--------------------------------------|--------------------------------------------|
| ! Normal view diagram with particles displayed | ☑ gas <br> ⬜ liquid <br> ⬜ solid <br> ⬜ element <br> ☑ compound <br> ⬜ mixture <br> ⬜ solution <br> ☑ pure substance <br> ⬜ homogeneous mixture <br> ⬜ heterogeneous mixture |
Diagram Explanation:
- The diagram visually represents gas particles. The configuration illustrates the particles dispersed evenly with space between them.
- Three magnification options are available: Normal, 1000X, and 10,000,000X, although only the normal view is shown here.
Checklist:
- The correct classifications in this case are "gas," "compound," and "pure substance."
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Höhörs Chemistry- 4th Hour - Dr. Paul / Gases/Lesson 148 What does the kinetic molecular theory tell us about the temperature of a substance? O The temperature of a substance does not affect the motion of the particles. OA substance with a lower temperature has particles that move faster. O A substance with a higher temperature has particles that move slower. O A substance with a higher temperature has particles that move faster.arrow_forwardOf the flasks or laboratory instruments that are given, express their function (specify -if they can be used to measure- if their measurements are approximate or relatively exact) Erlenmeyer flask beaker burettearrow_forwardThe following volumes of 0.000300 M SCN are diluted to 15.00 mL. Determine the concentration of SCN in each sample after dilution. These values will be used during the experiment. To enter exponential values, use the format 1.0e-5. Sample 0.000300 M SCN (mL) [SCN'] (M) 1 1.50 3.50 7.00 4 10.00 3.arrow_forward
- A hypothetical metal has a theoretical density of 11.47 g/cm3. This metal adopts a cubic crystal structure with an edge length of 0.387 nm and atomic radius of 0.137 nm. Find the atomic weight of this metal in g/mol. NA = 6.022 x 1023 atoms/mol. Express your answer in two decimal places only. Do not put the units.arrow_forwardIf a water sample has 0.07 mol of calcium ions in 15 L solution, what is the water hardness level in mg/L? Pay attention to the unit. Hint: For the calcium mass , convert mol to g to mg. (1 mol calcium ion= 40.08 g/mol and 1 g = 1000 mg) Round and report your answer to an integer without decimal place. Report numeric value only, no unit. • Water hardness levels according to EPA Soft: 0-60 milligrams per liter (mg/L) as calcium carbonate Moderately hard: 61-120 mg/L as calcium carbonate • Hard: 121-180 mg/L as calcium carbonate Very hard: more than 180 mg/L as calcium carbonatearrow_forward503.jpg ^ 1:50 molecular loval pictu 4. Ethanol (shown below) has a boiling point of 78 °C. When the temperature of ethanol is raised from room temperature to 78 °C, what is broken? Use a molecular level picture to clearly show this. H H H C C O H H Harrow_forward
- Explain your rationale for using the summative assessment (below) from the lesson plan on mixing substances in a 5th-grade classroom based on the below standard and learning objective, including how you will use student outcomes to inform future instructional decisions. Summative Assessment Summative: Components of the Lab Report Purpose: The student should clearly state the purpose of the experiment, which is to determine whether the mixing of two or more substances results in new substances. Procedure: The student should describe the steps they took to conduct the experiment. This includes the substances they chose to mix and the safety equipment they used. Observations: The student should record any observable changes that occurred when the substances were mixed. This could include a color change, the formation of a precipitate, or a change in temperature. Conclusion: The student should state whether a new substance was formed based on their observations. They should also…arrow_forwardSuppose some measurements are made on two different homogeneous stones to find out if they are made of the same kind of rock. The mass and volume measurements are listed below. Are the two stones the same type of rock? Why or why not? Show all calculations. Mass Volume Calculations Stone 1 58.0 g 20.0 cm Stone 2 50.1 g 15.0 cm3 21/common/assets/pdfjs/1.0.0.30/web/viewer.ht...ndered-pdf&fullscreen=Dd21-fileviewer-rendered-pdf-dialog&height=746#0arrow_forwardPercentage Abundance. The element rubidium consists of two isotopes, one having an isotopic mass of 84.912 and the other having an isotopic mass of 86.920. Determine the percentage abundance of the lighter isotope. Record the answer to the correct number of significant figures. Show the numerical set-ups. Include units and the correct number of significant figures in the set-up.arrow_forward
- 8. A chemist performs the same tests on the substances below. The results are recorded. Conductivity tests whether the following substances conduct electricity. A conductivity tester has a small bulb that can light up. Below are the results. Answer the questions that follow the chart. Conductivity Conductor Rating Tester Test Substance (good, poor, none) (Record light as off, dull, bright, or blinking) None Solid Salt Does not conduct. Solid Sugar Does not conduct. None Solid Wax Does not conduct. None Distilled Water Does not conduct. None Salt Water Conducts. Good Sugar Water Does not conduct. None Does not conduct. None Melted Waxarrow_forwardAnswer the third row (soap bubbles in the air). Please provide an explanation.arrow_forwardMixtures can be classified as either homogeneous or heterogeneous. Compounds cannot be classified in this way. Why not? In your answer,explain what is meant by heterogeneous and homogeneous.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY