
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Answer the 4 questions on the second pictured based on the data table provided. PPT means precipitate and NR is NO REACTION. POST PICTUREs OF YOUR WORK PLEASE.

Transcribed Image Text:1.
-200
POST-LAB
DOUBLE DISPLACEMENT REACTIONS
Name: Pich b
Write the name and formula of all the precipitates formed in this experiment. Designate which
well the precipitate was produced (ex: D1, aluminum carbonate, Al₂(CO₂),).
2. Which anion solution(s), left column of the data table, produced the fewest
insoluble compounds?
3. Which anion solution(s), left column of the data table, produced the largest number of
insoluble compounds?
4. Write out the net ionic reaction for each well that produced a precipitate on a separate sheet of
paper. Phases are not necessary.
Experiment 8 Double Displacement Reactions: Post-Lab
E
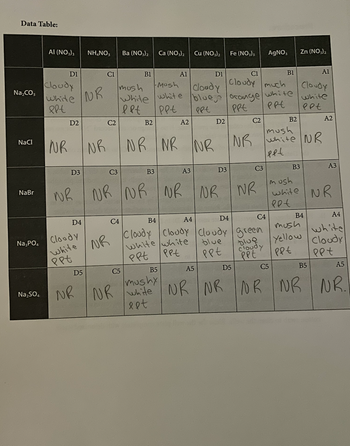
Transcribed Image Text:Data Table:
Na₂CO3
NaCl
NaBr
Na3PO4
Na₂SO4
AI (NO3)3 NH4NO3 Ba(NO3)2
D1
Cloudy
White NR
elt
NR
D2
D3
D4
Cloody
White
Ppt
NR
NR NR
D5
C1
NR
C2
C3
C4
mosh
White
let
C5
NR NR
B1
B2
B3
Ca (NO3)₂
A1
B4
Mush
white
pot
A2
NR NR NR
NR NR
A3
Cu (NO3)2
A5
B5
mushy
white NR
est
D1
Cloudy
bluege orange
ppt
Ppt
D2
NR
D3
Fe (NO3)3
ᎠᏎ
C1
Cloudy much
C2
D5
C3
A4
Cloudy Cloudy Cloudy green
white white
blue
Ppt
Ppt
Ppt
NR
C4
AgNO3
Dlug
cloudy
Ppt
mush
NR white NR
pet
white
PPE
NR NR
B1
C5
B2
B3
Zn (NO3)₂
Cloudy
white
eet
mush
Yellow
pet
B4
Al
mush
white NR
Pet
B5
A2
A3
A4
white
Cloudy
ppt
A5
NR NR.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Can You please answer question #4 that it is on the picture
(4. Write out the net ionic reaction for each well that produced a precipitate on a separate sheet of paper. Phases are not necessary.
Solution
by Bartleby Expert
Follow-up Question
can you please answer question #4 on th picture
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Can You please answer question #4 that it is on the picture
(4. Write out the net ionic reaction for each well that produced a precipitate on a separate sheet of paper. Phases are not necessary.
Solution
by Bartleby Expert
Follow-up Question
can you please answer question #4 on th picture
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- O science 24: Module 1 O bas ( 1 E Assignment Booklet 18 For questions 5 to 9, read each question carefully. Decide which of the choices BEST completes the statement or answers the question. Place your answer in the blank space given. 2 5. Iron oxide (FeO)) seperated into solid iron an example of a 1 (Fe (0)) A. simple composition reaction B. simple decomposition reaction C. combustion reaction D. neutralization reaction 6. In a simple composition reaction, A. one substance combines with another to form a salt and water B. two or more elements combine to form a compound C. a compound is broken down into two or more elements D. water is separated into hydroxgen gas and oxygen gas 7. fuel + oxygen → carbon dioxide + water vapour + energy This word equation is an example of a A. simple composition reaction B. simple decomposition reaction C. combustion reaction D. neutralization reaction A. simple composition reaction B. simple decomposition reaction C. combustion reaction D.…arrow_forwardPlease help with all partsarrow_forwardWhen answering this problem, report the answer with the appropriate number of significant figures. When entering units, use proper abbreviated units with proper capitalization. A student followed the procedure in this experiment, using 48.92 mL of 1.00 molar HCl and 51.08 mL of 1.00 molar NaOH. Analysis of the collected data resulted in a heat transfer of 2808 J during reaction. Use this information to determine the heat of neutralization for this reaction in kJ/mol. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: ___________*10___ Units______arrow_forward
- Part A Look at the three bottles. What evidence is there that a chemical reaction took place? Part B Compare bottles 1 and 2. How do the amounts of the reactants compare? How do the amounts of the products compare? For these two bottles, does the amount of product appear to be proportional to the amount of ammonia used? Part C Compare bottles 2 and 3. How do the amounts of the reactants compare? How do the amounts of the products compare? For these two bottles, does the amount of product appear to be proportional to the amount of ammonia used? Part D Compare your answers in parts B and C. If your answers to those questions are different, explain why they’re different. Part E Imagine mixing 1 tablespoon of Epsom salt with 2 cups of ammonia. How much precipitate would be produced? Describe the amount of precipitate by comparing it with the amount in bottle 1, 2, or 3. Explain your prediction. i need them all answered pleasearrow_forwardPLEASE HELParrow_forwardPredict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced. HI + NaOH → I Don't Know 1841 Submit 3 E 5 T 6 G 0-0 X S stv♫ 5 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cer A all 9 zoomarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY