
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
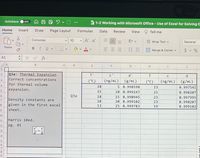
Need help finding correct concentrations for thermal volume expansion
Transcribed Image Text:3ORS
AutoSave
OFF
1-2 Working with Microsoft Office - Use of Excel for Solving C
Home
Insert
Draw
Page Layout
Formulas
Data
Review
View
O Tell me
Consolas
10
A A
22 Wrap Text v
General
Paste
BIU v
A
$ v %
Merge & Center v
A1
fx
A
C
2
Q3a: Thermal Expansion
c'
d'
d
Correct concentrations
(°C)
(ng/mL) (g/mL)
(°C)
(ng/mL) (g/mL)
for thermal volume
4
18
5 0.998598
23
0.997541
expansion.
15
10 0.999247
21
0.998207
Q3a
18
15 0.998945
23
0.997995
Density constants are
given in the first excel
18
20 0.999102
23
0.998207
8
13
25 0.999783
19
0.999102
sheet.
10
11
Harris 10ed.
12
pg. 41
13
14
15
11
17
8
on (ml)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. A gas consist of 70% propane and 30% butane by volume. Find the stoichiometric air-to-fuel ratio. a. 3:14 b. 14: 3 c. 23:1 d. 1:23 2. A furnace is fired with fuel oil with a partial analysis of 7.6% S and 2.8% N. Orsat analysis of the stack gas shows 9.44% CO2, 1.19% CO, 0.4% SO2, 0.47% H2, 6.8% O2 and 81.7% N2. Air supplied is at 23 deg Celsius, 755 mmHg and 85% RH. Calculate the percent excess air. a. 55% b. 78% c. 23% d. 38%arrow_forwardhelp with stoichometry bracketsarrow_forwardDiscuss the reason for potential differences between measured values of Chemical Oxygen Demand and Biochemical Oxygen Demand – ultimate for a given samplearrow_forward
- Recheck and Present in clear and complete readable typewritten or scanned solutionsarrow_forwardNitroglycerin is a dangerous powerful explosive that violently decomposes when it is shaken or dropped. The Swedish chemist Alfred Nobel (1833-1896) founded the Nobel Prizes with a fortune he made by inventing dynamite, a mixture of nitroglycerin and inert ingredients that was safe to handle. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of liquid nitroglycerin (C,H;(NO;),) into gaseous dinitrogen, gaseous dioxygen, gaseous water and gaseous carbon dioxide. 믐 x10 4C;H, (NO,), (1) → N, (3) + 0, (3) + CO,(8) + H,0(g) ? 2. Suppose 74.0L of carbon dioxide gas are produced by this reaction, at a temperature of - 13.0 °C and pressure of exactly 1 atm. Calculate the mass of nitroglycerin that must have reacted. Round your answer to 3 significant digits.arrow_forwardSolve correctly please. (Gpt/Ai wrong answer not allowed)arrow_forward
- (20u n. Illt Illix and the final pressure of the mixture. 13 - 36 A mixture of gases consists of 0.9kg of oxygen, 0.7kg of carbon dioxide, and 0.2kg of helium. This mixture is maintained at 100kPa and 27°C. Determine the apparent molecular weight of this mixture, the volume it occupies, the partial volume of the oxygen, and the partial pressure of the helium. Answers: 19.1kr 1k/mc mol,2.35m3, 0.702m3, 53.2kPa Determine this answers making a table and with the formulasarrow_forward"Nitric acid reacts with S to give H2SO4 and nitrogen monoxide. 30 ml of acid at 25% by mass and a density of 1.08 g/ml with 5 g of S. a) Adjust the reaction. b) Calculate limiting reactant and its excess. c) Calculate mass of unreacted reactant. d) Determine volume of NO at 600 mm Hg and 20°C. 20% yield"arrow_forward1. A group of students from the instrumental analysis laboratory of the course analyzed the sodium content in a serum sample using ICP-OES. They used 5 flasks with 25 mL of sample serum in each and added different amounts of a standard 2.640MNaCl solution to a total volume of 50 mL . Calculate: a. Equation for calibration b. Serum sodium concentrationarrow_forward
- Question 18 of 25 Submit How many liters of H2 gas would be produced by the complete reaction of 2.93 g of Al solid at STP according to the following reaction? Remember 1 mol of an ideal gas has a volume of 22.4 L at STP. 2 Al (s) + 6 HCI (aq) – 2 AICIS (aq) + |H2 (g) STARTING AMOUNT ADD FACTOR DELETE ANSWER RESET 2.02 6 2 2.93 3.65 3 1 1.62 6.022 x 1023 22.4 26.98 0.329 7.30 g/mol Al LAI g H2 mol H2 mol Al g/mol H2 LH2 g AI + 3. IIarrow_forwardshow full & complete procedure. Please answer parts a), b) & c). Note they are subparts of the same questionarrow_forwardHow much pressure is produced in kPa from the reactions of 2g, 4g and 6g of sodium bicarbonate and 50mL, 100mL and 150mL of 5% vinegar (acetic acid) in a 600mL container (9 reactions total). please show all of your working out.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY